Table 3.
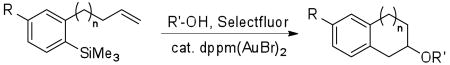 | |||||
|---|---|---|---|---|---|
| entry | R | R′ | n | product | yield (%) |
| 1 | H | H | 1 | 41 | 66 |
| 2 | Me | 1 | 42 | 73 | |
| 3 | Et | 1 | 43 | 70 | |
| 4 | H | 0 | 44 | 15c | |
| 5 | 4-F | H | 1 | 45 | 47 |
| 6 | Et | 1 | 46 | 68 | |
| 7 | 4-Cl | H | 1 | 47 | 62 |
| 8 | Me | 1 | 48 | 65 | |
| 9 | 4-CF3 | H | 1 | 49 | 51 |
| 10 | Me | 1 | 50 | 59 | |
| 11 | 4-Ph | Me | 1 | 51 | 74 |
Conditions: Aryltrimethylsilane (100 μmol), Selectfluor (200 μmol), 5 mol % dppm(AuBr)2, 0.1 M in CH3CN/R′OH (10/1), 50 °C for 14 h.
Catalyst addition in two portions over two hours.
Yield based on 1H NMR versus nitrobenzene as internal standard.
