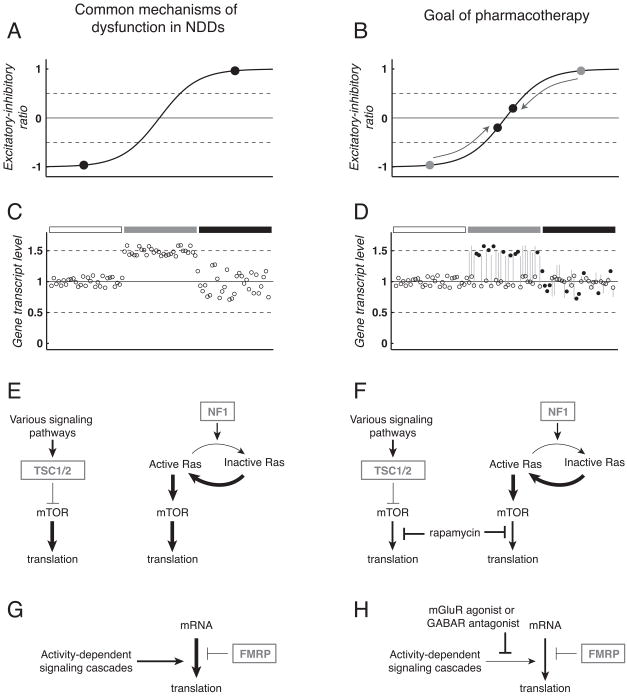
Figure 2. Neurodevelopmental disorders (NDDs) share common mechanisms of dysfunction amenable to similar therapeutic strategies.
A: Imbalanced excitation and inhibition within neuronal circuits occurs in a number of NDDs. Schematic representation of excitatory-inhibitory ratio shows pathophysiologically high (black circle, upper right) or low (black circle, bottom left) levels that exceed a theoretical window of balanced activity (between horizontal dashed lines). Note near balanced ratios are essential for normal circuit and cognitive function. B: Pharmacotherapy to address excitatory-inhibitory imbalance modifies neural circuits to achieve a level of activity that supports normal brain function (black circles, center). C: Abnormal gene dosage underlies dysfunction in many NDDs. Schematic representation of expression for several dozen genes under conditions of normal expression (left, under open rectangle), increased gene dosage for a number of genes as expected to occur in Down syndrome (center, under grey rectangle), and pseudo-realistic variability caused by gene-gene interactions and other factors (right, under black rectangle). D: Pharmacotherapy to address abnormal gene dosage may achieve normalization of altered transcript levels (center and right, under grey and black rectangles, open circles with grey lines to indicate pre-treatment expression). Improved cognitive function may occur without modifying expression of some genes (black circles). E: Dysregulated control of protein translation at synapses occurs in several NDDs. In tuberous sclerosis (TS), loss-of-function of the TSC1/2complex reduces inhibition of mTOR and leads to high levels of protein translation (left). Similarly, loss-of-function of NF1 drives increased translation via reduced inactivation of Ras in neurofibromatosis type 1 (NF-1) (right). F: In both TS and NF-1, drugs that inhibit mTOR-mediated inhibition such as rapamycin (Rapamune™ or sirolimus, Wyeth, Madison, NJ) are used to reduce translation to normal levels. G: In fragile X syndrome (FXS) activity-dependent signaling cascades drive translation at the synapse that is normally controlled by negative feedback from fragile X mental retardation protein (FMRP). H: Potential therapeutic strategies for FXS may suppress activity-dependent signals to restore normal control of translation in the absence of FMRP. In panels E–H, arrows and T-bars indicate activation and inhibition of signaling pathways, respectively, arrow and T-bar thickness represent the strength of activation or inhibition, and boxed grey text corresponds to genes absent or mutated in specific NDDs. For brevity, many components of signaling cascades have been excluded. Moreover, it should be noted that Ras, mTOR, FMRP and mGluR signaling are not independent from each other, further supporting the concept that seemingly distinct genetic lesions in NDDs converge on critical regulatory pathways to alter synaptic, circuit and cognitive function. Abbreviations: mammalian target of rapamycin (mTOR); tuberous sclerosis gene products tuberin and hamartin (TSC1/2); neurofibromatosis type 1 gene product neurofibromin (NF1); group 1 metabotropic glutamate receptor (mGluR); GABA receptor (GABAR).