Abstract
BACKGROUND & AIMS
Helicobacter pylori-induced immune responses fail to eradicate the bacterium. Nitric oxide (NO) can kill H. pylori. However, translation of inducible NO synthase (iNOS) and NO generation by H. pylori-stimulated macrophages is inhibited by the polyamine spermine derived from ornithine decarboxylase (ODC), and is dependent on availability of the iNOS substrate L-arginine (L-Arg). We determined if spermine inhibits iNOS-mediated immunity by reducing L-Arg uptake into macrophages.
METHODS
Levels of the inducible cationic amino acid transporter (CAT)2, ODC, and iNOS were measured in macrophages and H. pylori gastritis tissues. L-Arg uptake, iNOS expression, and NO levels were assessed in cells with siRNA knockdown of CAT2 or ODC, and in gastric macrophages. The ODC inhibitor, α-difluoromethylornithine (DFMO), was administered to H. pylori-infected mice for 4 months post-inoculation.
RESULTS
H. pylori induced CAT2 and uptake of L-Arg in RAW 264.7 or primary macrophages. Addition of spermine or knockdown of CAT2 inhibited L-Arg uptake, NO production, and iNOS protein levels, whereas knockdown of ODC had the opposite effect. CAT2 and ODC were increased in mouse and human H. pylori gastritis tissues and localized to macrophages. Gastric macrophages from H. pylori-infected mice exhibited increased ODC expression, and attenuated iNOS and NO levels upon ex vivo H. pylori stimulation versus cells from uninfected mice. DFMO treatment of infected mice restored L-Arg uptake, iNOS protein expression, and NO production in gastric macrophages, and significantly reduced both H. pylori colonization levels and gastritis severity.
CONCLUSIONS
Upregulation of ODC in gastric macrophages impairs host defense against H. pylori by suppressing iNOS-derived NO production.
Keywords: spermine, macrophages, gastritis
Introduction
Helicobacter pylori is a Gram-negative, microaerophilic bacterium that colonizes the human gastric mucosa. It infects about half of the world’s population, and ~30–40% in the United States.1,2 H. pylori induces an innate immune response in neutrophils, monocytes, and macrophages3–7 and a chronic lymphocytic response.8 While H. pylori is classically considered a noninvasive pathogen, the bacterium disrupts epithelial integrity and both the bacterium itself9 and its antigens3 are present in the lamina propria, and are in direct contact with immune cells where they can impact responses in macrophages and other antigen-presenting cells.8,9 However, the overall immune response is ineffective, as the bacterium generally persists for the life of the host, leading to chronic gastritis, peptic ulcers, lymphoma, and gastric adenocarcinoma, the second leading cause of cancer death worldwide.1 Therefore, gaining insight into the failure of the immune response could be of great importance in developing new strategies for chemoprevention or disease management.
Nitric oxide (NO) is a fundamental component of innate immunity with well-characterized antimicrobial and antitumor effector functions.6,10–13 High-output production of NO derives from the substrate L-arginine (L-Arg) by inducible NO synthase (iNOS, NOS2), which defines the classical pathway of activation in macrophages by microbes and their products, and is of critical importance to host defense.6,10,14 We have demonstrated that H. pylori induces iNOS and NO production in macrophages in vitro and that this effect is contact-independent, occurring with H. pylori lysates, soluble surface proteins, culture supernatants, and recombinant urease.4–6,15,16 In addition, we have reported that NO generation in H. pylori-stimulated macrophages is strongly dependent on L-Arg availability in culture medium and requires concentrations well above the circulating physiologic level of L-Arg in mice and humans of 0.1 mM to maximize response; this effect was mediated primarily by an enhancement of iNOS translation with increasing L-Arg levels.16 Further, in our studies, macrophages kill H. pylori in an NO-dependent manner, but this requires concentrations of L-Arg of 0.4 mM and greater.6,15,16 Consistent with our findings, it has been demonstrated that L-Arg can increase NO generation by iNOS, even at concentrations greatly above the KM of the enzyme,10 which is in the range of 10 μM.11
iNOS is expressed in gastritis tissues of H. pylori-infected humans, and localizes to inflammatory cells, particularly macrophages.17,18 However, iNOS is unable to generate an antimicrobial benefit for the infected host. The adaptive immune response is also insufficient, since enhancement of Th1/Th17 responses in adoptive transfer and vaccine studies reduces H. pylori colonization.8,19,20 We have reported that one mechanism that restricts H. pylori-stimulated NO generation in macrophages is the simultaneous generation of polyamines within these cells by upregulation of ornithine decarboxylase (ODC).5,15,21,22 ODC converts L-ornithine into putrescine, which is metabolized into the polyamines spermidine and spermine by constitutively expressed synthases. We have reported that spermine acts to inhibit NO production and killing of H. pylori by reducing stimulated iNOS protein expression through an effect on iNOS translation; spermidine had a more modest inhibitory effect on iNOS, while putrescine had no such effect.15 As with iNOS,23 the induction of ODC occurs by soluble factors and is attenuated by isogenic deletion of UreA and recapitulated by recombinant urease.5,15,22
L-Arg uptake in macrophages has been attributed to the y+ transport system.12 The cationic amino acid transporters (CAT) include CAT1, which is constitutively expressed and involved in uptake of L-Arg for basic metabolism,13 and CAT2, which includes the alternatively spliced isoforms CAT2A, a low affinity transporter primarily in liver, and CAT2B the high affinity L-Arg transporter in macrophages.12,13,24,25 The CAT2 protein is part of a larger family of solute carriers, and is thus also known as solute carrier 7 (SLC7)A2.24 CAT3 and CAT4 have also been described.14,24 CAT3 is found in brain and thymus, and CAT4 function is unknown at this time.14,24 We now report that H. pylori-induced CAT2 expression mediates L-Arg uptake, iNOS protein synthesis, and NO production, and that spermine inhibits iNOS by blocking L-Arg transport into macrophages. CAT2 and ODC are both upregulated in macrophages within H. pylori gastritis tissues. Administration of an ODC inhibitor to H. pylori-infected mice enhanced L-Arg uptake, iNOS protein levels, and NO production in gastric macrophages, and resulted in significant attenuation of both H. pylori colonization and gastritis. Therefore, modulation of polyamine generation in the stomach could represent a novel approach to enhancing immunity to H. pylori.
Materials and Methods
Reagents
Reagents for cell culture, RNA extraction, and RT-PCR were from Invitrogen. Other chemicals were from Sigma. α-difluoromethylornithine (DFMO) was from ILEX Oncology (San Antonio, TX).
Bacteria
H. pylori SS1, C. rodentium, and C. jejuni were used as in Supplementary Methods.
Mice, Cells, and Culture Conditions
The murine macrophage RAW 264.7 cell line was maintained as described.4,5,15,21 In C57BL/6 mice, peritoneal macrophages were isolated15, gastric infection performed,5,26 and colonization and gastritis assessed (Supplementary Methods). Some mice were treated with DFMO (1% w/v) in the drinking water.27,28
Human Subjects
Tissues from endoscopic biopsies were utilized as described.17
Measurement of NO
The concentration of nitrite (NO2−), the oxidized metabolite of NO, was assessed by the Griess reaction.4–6,15
RT-PCR
RNA was extracted using TRIzol and cDNA synthesized.5,16 Primer sequences and PCR methods are in Supplementary Methods.
L-Arg Transport
Immunoblotting for iNOS and CAT2
Western blotting for iNOS and β-actin was performed as described.15,16 CAT2 was detected with a rabbit polyclonal antibody to the C-terminal 70 amino acids of mouse CAT2.12
Transient Transfection of CAT2 and ODC siRNA
Transfection with siRNA duplexes was performed as described;15,21 sequences are in Supplementary Methods.
ODC Activity
ODC enzyme activity was measured by radiochemical assay.27
Immunohistochemistry for ODC and CAT2, and Immunofluorescence Staining for CAT2 and the Macrophage Marker F4/80
Polyamine Measurement
Polyamines were measured as described.27
Gastric Macrophages
Cells were isolated from glandular stomach by positive selection with F4/80 antibody.16,29 RNA isolation and real-time PCR, and flow cytometry were performed as described.16,29
Statistical Analysis
Quantitative data are shown as the mean ± SE. Analyses are in Supplementary Methods.
Results
H. pylori Stimulates L-Arg Uptake in Macrophages That Is Inhibited by Spermine
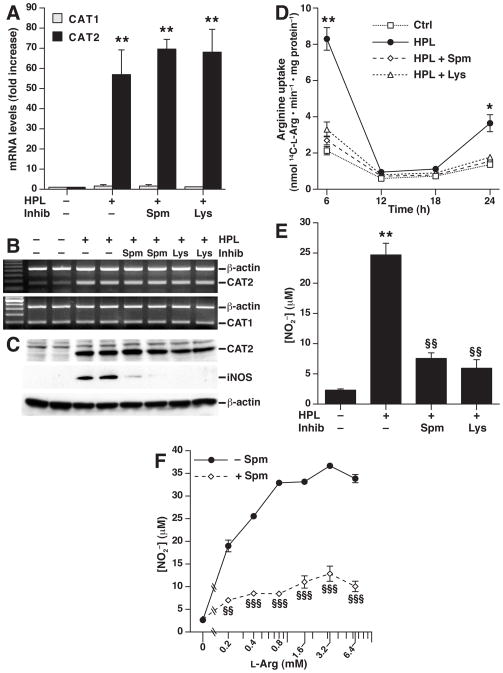
When RAW 264.7 cells were stimulated with H. pylori lysate, CAT2 mRNA levels were increased by >50–fold when assessed by real-time PCR, while CAT1 was not induced (Fig. 1A). Neither spermine, nor L-lysine (L-Lys), a known competitive inhibitor of L-Arg uptake,25 had any effect on stimulated CAT2 mRNA levels (Fig. 1A and 1B). Similarly, H. pylori upregulated CAT2 protein levels, as assessed by Western blotting, and spermine and L-Lys did not affect CAT2 protein expression (Fig. 1C). In contrast, both spermine and L-Lys markedly attenuated iNOS protein levels (Fig. 1C), consistent with our previous reports.15,16
Figure 1.
Induction of CAT2 and L-Arg uptake in RAW 264.7 macrophages and effect of spermine and L-lysine. Macrophages were incubated with H. pylori lysate (HPL) at an MOI of 100. mRNA expression for CAT1 and CAT2 was assessed at 6 h after HPL stimulation ± the inhibitor (Inhib) spermine (Spm; 12.5 μM) or L-lysine (Lys; 20 mM) by real-time PCR (A) and semi-quantitative RT-PCR (B). (C) Western blot analysis for CAT2 (80 kDa), iNOS (130 kDa) and β-actin (42 kDa) 24 h after stimulation. (D) L-Arg transport assessed as uptake of 14C-L-Arg into cells at the times indicated. (E and F) NO levels measured as concentration of NO2−, at 24 h after stimulation. In (F), L-Arg was added to L-Arg-free, serum-free medium ± Spm. In A–F, data are from 3 experiments performed in duplicate. For A and E, **P < .01 vs control; for D, *P < .05, **P < .01 vs control, HPL + Spm, and HPL + Lys; for E and F, §§P < .01, §§§P < .001 vs HPL alone.
Because uptake of extracellular L-Arg has been shown to be required for iNOS-derived NO generation in macrophages13,16,25 we assessed L-Arg transport from the extracellular medium into macrophages. H. pylori stimulation resulted in a 4–fold increase in L-Arg uptake by RAW 264.7 cells, which was blocked by addition of either spermine or L-Lys (Fig. 1D). Spermine and L-Lys each inhibited H. pylori-stimulated NO production (Fig. 1E) in a manner that paralleled their effects on L-Arg uptake. These findings raised the possibility that spermine could inhibit LArg uptake by competing for the same transporter. However, addition of increasing levels of LArg did not overcome the inhibitory effect of spermine on NO production, even at 6.4 mM (Fig. 1F), which is 64–fold greater than the circulating level of 0.1 mM in mice and humans.27,30
When primary mouse peritoneal macrophages were used, H. pylori-stimulated cells also exhibited induction of CAT2, but not CAT1 mRNA levels (Supplementary Fig. 1A), and a significant increase in L-Arg uptake with H. pylori stimulation that was abolished by spermine (Supplementary Fig. 1B). These data are consistent with the inhibitory effect of spermine on NO generation in peritoneal macrophages that we have reported.15
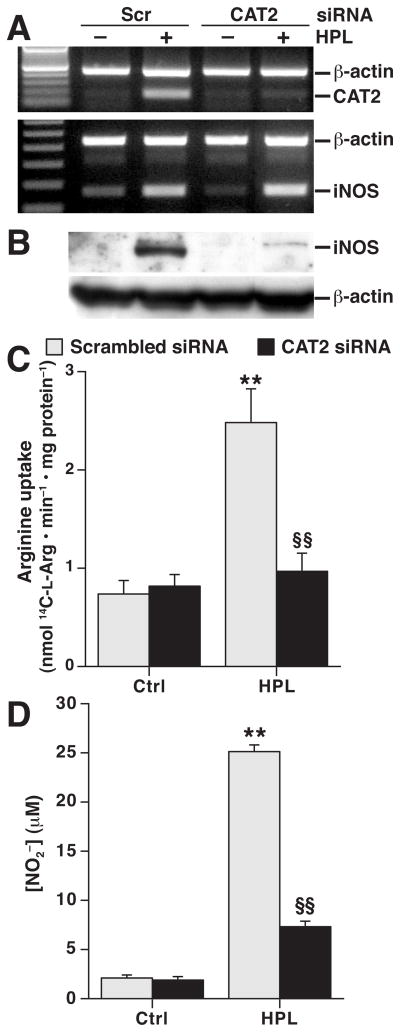
H. pylori-stimulated iNOS Protein Expression and NO Production in Macrophages Is Dependent on Induction of CAT2
The role of CAT2 in NO production has not been previously assessed with an extracellular bacterial stimulus. Therefore, CAT2 knockdown by siRNA was performed in H. pylori-stimulated cells (Fig. 2A). While this knockdown had no effect on iNOS mRNA levels (Fig. 2A), it resulted in a marked inhibition of iNOS protein expression (Fig. 2B), which was paralleled by reduced L-Arg uptake (Fig. 2C) and NO generation (Fig. 2D). Consistent with our report that L-Arg availability regulates iNOS translation and not protein stability,16 inhibition of iNOS protein degradation with the proteosomal inhibitor lactacystin did not restore iNOS protein levels in the presence of CAT2 siRNA at time points from 6 to 24 h (Supplementary Fig. 2).
Figure 2.
Effect of transfection of CAT2 siRNA on H. pylori-stimulated iNOS expression and NO production. RAW 264.7 cells were transiently transfected with either scrambled siRNA or siRNA to CAT2. (A) mRNA expression assessed by RT-PCR at 6 h after stimulation with HPL. (B) Western blot analysis at 24 h after stimulation. In A and B, n = 3. (C) L-Arg uptake and (D) NO2− levels, measured 24 h after stimulation. **P < .01 vs control macrophages transfected with scrambled siRNA; §§P < .01 vs HPL-stimulated macrophages transfected with scrambled siRNA, n = 6. Scr, scrambled.
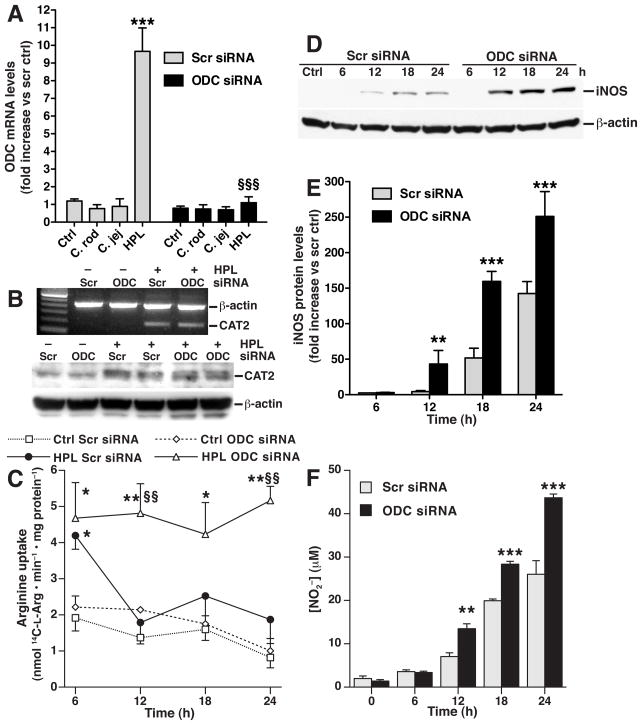
Knockdown of ODC Increases iNOS-derived NO Production by Causing Sustained L-Arg Uptake
We next determined the effect of endogenous spermine generation on CAT2, L-Arg uptake, and iNOS. We have reported that transient transfection of ODC siRNA results in effective decreases in ODC expression, activity, and spermine levels in H. pylori-stimulated RAW 264.7 macrophages.15 We verified (Fig. 3A) that H. pylori lysate induced a 9–fold increase in ODC mRNA levels in cells transfected with scrambled siRNA, while lysates of the enteric pathogens C. jejuni and C. rodentium failed to induce ODC; additionally, ODC siRNA abolished H. pylori-stimulated ODC expression. ODC siRNA transfection did not affect induction of CAT2 mRNA or protein levels (Fig. 3B). However, with ODC knockdown the H. pylori-stimulated L-Arg uptake at 6 h persisted out to 24 h, whereas cells transfected with scrambled siRNA had attenuation of L-Arg uptake beyond the first 6 h after activation (Fig. 3C). There was more iNOS protein expression detected at 12 – 24 h after H. pylori stimulation in the presence of ODC siRNA than in cells transfected with scrambled siRNA (Fig. 3D). These findings were confirmed by densitometry (Fig. 3E). In parallel, ODC knockdown increased NO generation from 12 to 24 h after activation (Fig. 3F). ODC knockdown also enhanced L-Arg uptake and NO production in macrophages activated with live H. pylori (Supplementary Fig. 3). This effect was not observed when cells were activated with C. jejuni or C. rodentium (Supplementary Fig. 4).
Figure 3.
Transfection of ODC siRNA enhances L-Arg uptake and iNOS protein expression in RAW 264.7 macrophages. (A) Real-time PCR for ODC with stimulation with lysates of C. rodentium (C. rod), C. jejuni (C. jej) and HPL all at an MOI of 100. (B) top panel, RT-PCR for CAT2; bottom panel, Western blot analysis for CAT2 and β-actin. (C) L-Arg transport (Scr, scrambled). *P < .05, **P < .01 vs control macrophages transfected with scrambled siRNA; §§P < .01 vs HPL-stimulated macrophages transfected with scrambled siRNA. (D) Western blot analysis for iNOS and β-actin. (E) Densitometry of iNOS Western blots, standardized to β-actin. (F) NO2− levels measured at 24 h after stimulation. In E and F, **P < .01; ***P < .001 vs macrophages transfected with scrambled siRNA. n = 3–4 for all experiments.
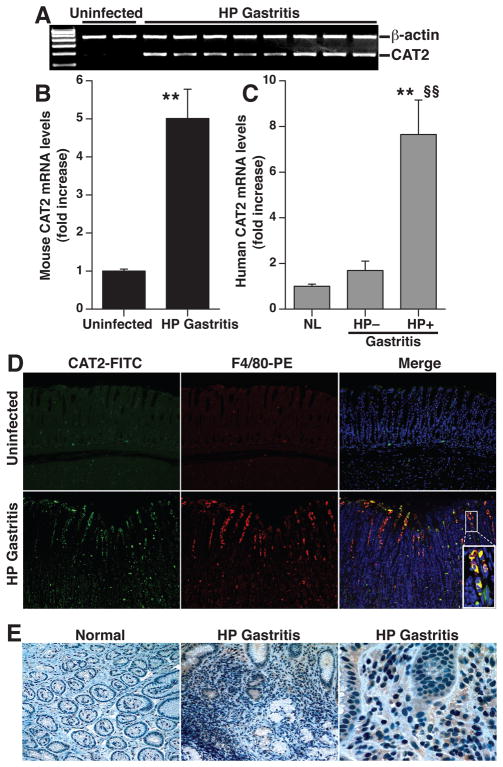
H. pylori Infection Causes Induction of CAT2 and ODC In Vivo
To provide further biological relevance, we determined the expression of CAT2 and ODC in H. pylori gastritis tissues. There was a consistent upregulation of CAT2 mRNA levels (Fig. 4A and 4B) in mouse chronic gastritis tissues. We also found an 8–fold increase in CAT2 mRNA levels in human H. pylori gastritis tissues compared to normal gastric tissues, while CAT2 was not upregulated in gastritis tissues from subjects without H. pylori infection (Fig. 4C). CAT2 protein levels were increased in infected mouse tissues when assessed by immunofluorescence, with co-localization to gastric macrophages in the mucosa and submucosa (Fig. 4D). Immunoperoxidase staining demonstrated increased CAT2 levels in human H. pylori gastritis tissues that localized to lamina propria mononuclear cells, with sporadic staining of some gastric epithelial cells (Fig. 4E).
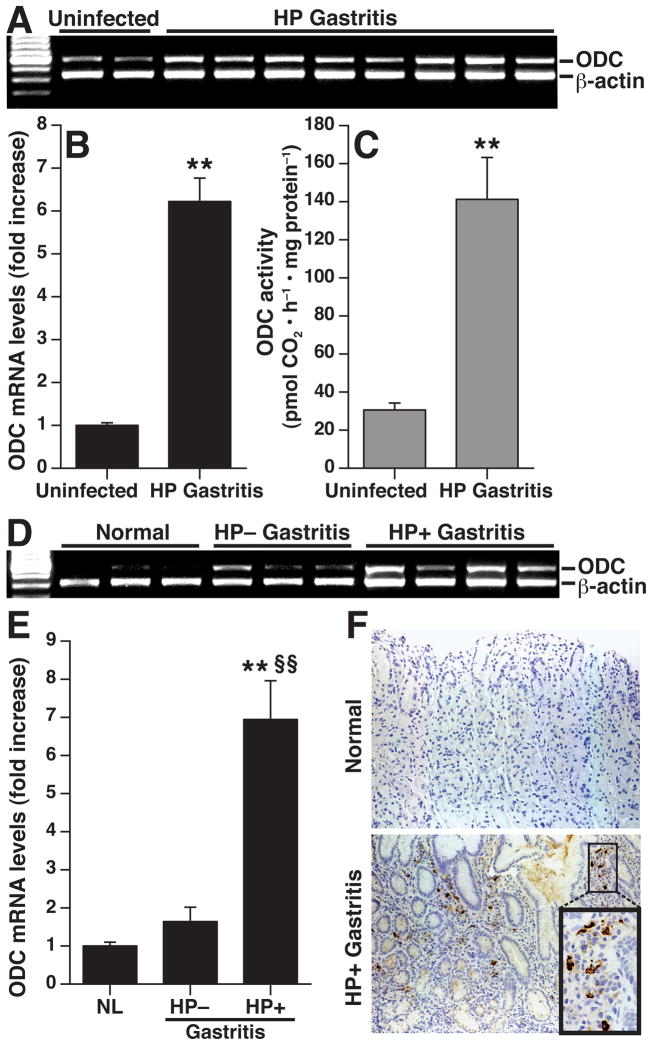
Figure 4.
Induction of CAT2 in mouse and human H. pylori gastritis tissues. (A) RT-PCR for CAT2 in mouse tissues from uninfected and H. pylori-infected mice at 4 months post-inoculation. (B) Real-time PCR for CAT2 in mouse tissues, n = 4 control and 9 infected. **P < .01 vs uninfected tissues (C) CAT2 expression by real-time PCR in human tissues from uninfected patients with normal histology (n = 4), H. pylori-negative gastritis (n = 4), and H. pylori-positive gastritis (n = 6). **P < .01 vs normal; §§P < 0.01 vs H. pylori-negative gastritis tissues. (D) Immunofluorescent detection of CAT2 that localizes to macrophages. Mouse tissues were stained for CAT2 detected with FITC (green) and for the macrophage marker F4/80, detected with TRITC (red). Nuclei were stained with DAPI (blue). Co-localization is demonstrated in merged images by yellow color. Magnification for each image is 200X, plus 600X for inset. (E) Immunohistochemistry for CAT2 in human biopsies, magnification is 200X in left and center panels, and 600X in right panel.
We also detected a significant increase in ODC mRNA levels in gastritis tissues from H. pylori-infected mice compared to uninfected control tissues (Fig. 5A and 5B). ODC enzyme activity was increased in parallel in the H. pylori gastritis tissues (Fig. 5C). There was also a significant increase in ODC mRNA expression in human H. pylori gastritis tissues (Fig. 5D and 5E), which was not observed with H. pylori-negative gastritis tissues. By immunohistochemistry, ODC was primarily expressed in mononuclear cells of the lamina propria in H. pylori-infected human gastritis tissues (Fig. 5F). Taken together, these data indicate CAT2 and ODC are upregulated in parallel in H. pylori gastritis.
Figure 5.
Induction of ODC in mouse and human H. pylori gastritis tissues. At 4 mo post- inoculation in mice, ODC was assessed by (A) RT-PCR, (B) real-time PCR, and (C) enzyme activity. **P < .01 vs uninfected. n = 4 uninfected and 9 infected in (B), and n = 5 uninfected and 5 infected in (C). In humans, ODC was assessed by (D) RT-PCR, (E) real-time PCR, and (F) immunohistochemistry. In (E), **P < .01 for H. pylori positive gastritis (n = 6) vs uninfected normal tissues (n = 4); §§P < 0.01 vs H. pylori-negative gastritis tissues (n = 4). In (F) magnifications are 200X for both normal and H. pylori-positive gastritis, and 600X in the inset.
Gastric Macrophages Isolated from H. pylori-infected Mice Exhibit Impaired Uptake of L-Arg, iNOS Protein Expression, and NO Production
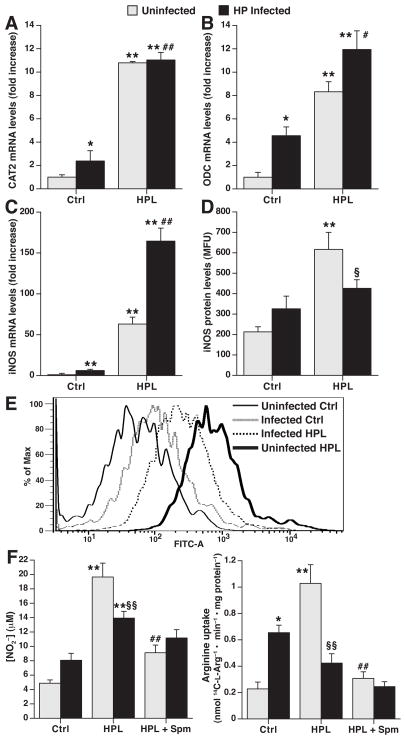
In order to assess the interplay between ODC, CAT2, and iNOS in vivo, we studied macrophages isolated from mice chronically infected with H. pylori at 4 months post-inoculation versus uninfected controls. These cells were then cultured in the presence or absence of H. pylori lysate ex vivo, to determine if ODC induction during gastritis regulates iNOS. CAT2 mRNA levels were increased in cells from infected mice (Fig. 6A), and upon ex vivo activation with H. pylori lysate there was a further increase in CAT2 levels in cells from either uninfected or H. pylori-infected mice. In the case of ODC (Fig. 6B) and iNOS (Fig. 6C), there was an increase in mRNA levels in macrophages from infected mice when compared to cells from uninfected mice, and with ex vivo stimulation with H. pylori lysate, the cells from infected mice exhibited a potentiation of mRNA levels. Because there are < 105 macrophages per stomach,29 flow cytometry was used to analyze protein levels; this demonstrated that iNOS was not significantly increased in cells from H. pylori-infected mice, and upon ex vivo stimulation these cells expressed less iNOS protein than cells from naïve uninfected mice (Figs. 6D and 6E). In parallel, there was a lack of significant increase in NO release from gastric macrophages from H. pylori-infected mice, and a decrease in NO production with ex vivo H. pylori stimulation when compared to cells from uninfected mice (Fig. 6F). When exogenous spermine was added, this significantly attenuated ex vivo-stimulated NO production in cells from uninfected mice, but there was little effect on cells from infected mice.
Figure 6.
Deficient iNOS protein expression and NO production in gastric macrophages from H. pylori infected mice. Mice were inoculated with H. pylori or broth control, and after 4 mo gastric macrophages were isolated by positive selection. Cells from both uninfected (light bars) and H. pylori-infected (black bars) mice were then treated ex vivo with vehicle control (Ctrl) or HPL for 24 h. (A–C) Real-time PCR analysis of CAT2, ODC, and iNOS mRNA levels respectively. (D), iNOS protein levels determined by flow cytometry; data are composite results in relative fluorescence units. (E) Representative histogram tracings for the conditions shown in D. In A–D, *P < .05, **P < 0.01 vs unstimulated control cells from uninfected mice; #P < .05, ##P < .01 for HPL-stimulated cells from infected mice vs unstimulated cells from infected mice; §P < .05 for HPL-stimulated cells from infected mice vs HPL-stimulated cells from uninfected mice, n = 6. (F) NO2− levels (left panel) and L-Arg uptake (right panel). Spm (12.5 μM) was added at the start of the ex vivo stimulation. *P < .05, **P < .01 vs unstimulated control cells from uninfected mice; §P < .05, §§P < .01 for HPL-stimulated cells from infected mice vs HPL-stimulated cells from uninfected mice; ##P < .01 for stimulation with HPL + Spm vs HPL only in cells from uninfected mice; n = 6.
L-Arg uptake (Fig. 6F) was modestly increased in cells from infected mice, and was substantially decreased in gastric macrophages from infected mice versus uninfected mice upon H. pylori stimulation ex vivo, consistent with the higher levels of ODC in these cells (Fig. 6B). Spermine reduced ex vivo H. pylori-stimulated L-Arg uptake to basal levels in the cells from uninfected mice, but did not further decrease L-Arg uptake in cells from infected mice. These data suggest that induction of ODC in macrophages during chronic H. pylori infection may explain a defective NO response to H. pylori due to limited L-Arg uptake and iNOS protein expression.
In Vivo Inhibition of ODC Restores NO-mediated Immunity to H. pylori and Ameliorates Gastritis
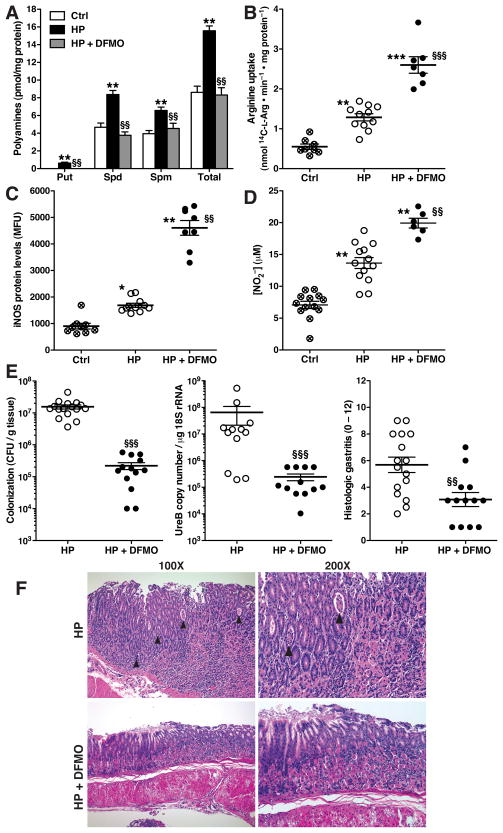
To directly determine if spermine accumulation attenuates NO-dependent innate immune response to H. pylori, we administered the ODC inhibitor DFMO to mice (1% w/v in the drinking water) for the 4-month course of infection, based on studies that have established chronic use of this agent for chemoprevention of colon tumors in mice.31 With H. pylori infection, there was a 2–fold increase in polyamine levels in gastric tissues that were reduced to basal levels with DFMO treatment (Fig. 7A). In freshly isolated gastric macrophages from H. pylori-infected mice, the levels of L-Arg uptake (Fig. 7B), iNOS protein (Fig. 7C), and NO (Fig. 7D) were significantly enhanced in cells from mice treated with DFMO. Importantly, direct assessment of gastric colonization with H. pylori revealed that DFMO treatment of mice resulted in a significant, 2-log-order reduction in bacterial levels, when assessed by culture or quantitative PCR (Fig. 7E). In parallel, there was a significant reduction in gastric inflammation, with no development of gastric atrophy, as demonstrated by histologic gastritis scores (Fig. 7E) and representative histologic sections (Fig. 7F).
Figure 7.
Inhibition of polyamine synthesis restores L-Arg uptake and iNOS-derived NO production, and reduces H. pylori colonization and gastritis. Mice were infected with H. pylori or broth control, and received DFMO (1% w/v) in the drinking water or water alone for 4 mo, beginning the day after inoculation with H. pylori. (A) Polyamine levels in stomach tissues. (B–D) Gastric macrophages were isolated from the groups indicated. L-Arg uptake (B), iNOS protein expression by flow cytometry (C), and NO2− levels (D) were assessed. In A–D, *P < .05, **P < .01, ***P < .001 vs uninfected control; §§P < .01, §§§P < .001 vs H. pylori-infected without DFMO. n = 6 mice per group in A, and in B, n = 6–13 per group; each symbol represents a different mouse. (E) H. pylori colonization and level of gastritis. Left panel, colonization by serial dilution and culture; center panel, quantitative PCR for ureB; right panel, histology scores for gastritis severity. Histologic scores in uninfected mice were 0.8 ± 0.3 (n = 14) and 0.7 ± 0.3 (n = 5), in mice receiving water alone or DFMO, respectively. §§P < .01, §§§P < .001 vs HP without DFMO. (F) Representative hematoxylin and eosin staining of gastritis tissues. Arrowheads indicate crypt abscesses.
Discussion
In experimental H. pylori infection there is a rapid innate immune response with influx of macrophages and neutrophils within 48h of inoculation.32 However, persistence of the bacterium has been attributed to defective adaptive immune responses, since decreased colonization has been demonstrated with exaggerated Th1 responses in adoptive transfer models19 or enhancement of Th1/Th17 responses in vaccine models.20 The ineffectiveness of the adaptive response has been related to enhanced regulatory T cell responses, which may protect the host from uncontrolled inflammation, but lead to survival of H. pylori.33 We determined if the function of macrophages in the innate response to H. pylori infection is compromised by impairment of L-Arg uptake that is essential for M1 response.14 We demonstrate that in H. pylori infection, the inducible L-Arg transporter CAT2 is upregulated, but its ability to transport L-Arg in macrophages is inhibited by ODC, resulting in loss of NO-derived immune defense against H. pylori (summarized in Supplementary Fig. 5.)
Our data indicate that CAT2 is the primary L-Arg transporter in H. pylori-activated macrophages, since there was a large induction of CAT2, and not CAT1, and knockdown of CAT2 prevented stimulated L-Arg uptake. Mycobacterium bovis similarly induces L-Arg transport in RAW 264.7 macrophages that is associated with CAT2, but not CAT1 expression.34 In macrophages from CAT2-deficient mice there is deficient NO production and L-Arg transport in response to IFN-γ plus lipopolysaccharide.13,14
We found that spermine inhibited L-Arg uptake in H. pylori-stimulated RAW 264.7 or primary peritoneal macrophages, without altering CAT2 mRNA or protein levels. There is a report of inhibition of CAT2 mRNA expression by spermine in LPS-stimulated alveolar macrophages, but the partial inhibition of CAT2 mRNA did not correlate with a more substantial effect of spermine on L-Arg uptake.35 There is also evidence of spermine inhibiting transport activity of a potassium channel without altering its expression.36 We have reported that H. pylori-induced NO production depends on availability of extracellular L-Arg, which enhances iNOS translation and thus iNOS protein synthesis.16 Extracellular spermine can be transported into macrophages within 2 h of stimulation by LPS.37 However, our data show that spermine is not a competitive inhibitor of L-Arg transport, in contrast to L-Lys.25 Spermine is able to bind to and inactivate proteins,38 raising the possibility that a similar effect could be occurring with CAT2 in H. pylori-stimulated cells.
We observed a biphasic effect of H. pylori on macrophage uptake of L-Arg, with increases at 6 and 24 h after stimulation, but no increase at 12 or 18 h. Because we also show that spermine inhibits L-Arg transport, this biphasic time course of L-Arg uptake is consistent with our previous report that ODC expression and enzyme activity peak 6 h after H. pylori stimulation, and spermine levels peak at 12 h, followed by a decrease out to 24 h.21,22 We now show that ODC knockdown resulted in a sustained increase in L-Arg uptake without this biphasic pattern, resulting in a concomitant increase in iNOS protein expression and NO production at time points from 12 – 24 h. These data indicate that endogenous synthesis of spermine by the induction of ODC impairs macrophage L-Arg transport in H. pylori infection. Spermine may also regulate its own production by inhibiting L-Arg uptake, since L-Arg can be rate-limiting for synthesis of the ODC substrate L-ornithine by arginase.14 These effects were specific to H. pylori, since C. jejuni and C. rodentium did not induce ODC, and ODC knockdown had no effect on L-Arg transport or NO production induced by these pathogens.
An important consideration is the in vivo significance of CAT2 and ODC in the infected stomach. CAT2 mRNA expression is increased in models of lung injury.39 CAT2 has not been previously studied in stomach; our data show upregulation of CAT2 expression and macrophage localization in H. pylori gastritis tissues. It has been reported that H. pylori eradication decreases ODC mRNA expression and activity,40 but the cellular source of ODC has not been identified. We now show that ODC expression is specifically increased in human H. pylori infection compared to H. pylori-negative gastritis or normal tissues and that experimental infection induces ODC expression. Immunostaining in human tissues revealed staining of lamina propria mononuclear cells, and we have demonstrated increased expression of ODC in gastric macrophages from infected mice. It has been reported that there are increased polyamine levels in gastric tissues from H. pylori-infected human subjects41 and that spermine levels were increased in intestinal-type tumors, typical of H. pylori-induced cancer, compared to diffuse gastric tumors.42
We have found that in isolated mouse gastric macrophages from H. pylori-infected mice there is a minimal increase in iNOS protein and NO production compared to cells from uninfected mice. There was also loss of induction of iNOS protein and NO synthesis, and diminished L-Arg uptake upon ex vivo stimulation with H. pylori in macrophages from chronically-infected mice. These data suggest a form of tolerance in innate immune response, with failure to mount an iNOS-mediated antimicrobial defense. This provides new insight into findings that iNOS-deficient mice do not exhibit an exacerbation of H. pylori colonization and that serum nitrate/nitrate is not increased in infected wild-type mice.43 Consistent with our data implicating ODC as an inhibitor of iNOS synthesis in vitro, we show that depletion of endogenous polyamine synthesis by DFMO enhanced the levels of L-Arg uptake, iNOS protein, and NO in gastric macrophages from H. pylori-infected mice. The importance of these data was confirmed by the ability of DFMO to reduce H. pylori colonization, as well as gastritis. This differs from reports that reduction in colonization requires increased gastritis.19,20,33 Our findings raise the possibility that DFMO could have a direct effect on the bacterium itself. However, H. pylori does not possess ODC,44 making it unlikely that the effect of DFMO occurs by altering H. pylori polyamine synthesis. Furthermore, we have found that: 1) H. pylori does not exhibit ODC enzymatic activity, in contrast to E. coli; 2) DFMO treatment of H. pylori in liquid culture does not impair bacterial viability; 3) the beneficial effect of DFMO on H. pylori colonization and gastritis is effectively attenuated in iNOS knockout mice (data not shown). The reduction in colonization with DFMO is consistent with our findings that killing of extracellular H. pylori by macrophages is dependent on NO and regulated by L-Arg availability.6,15,16 However, it is possible that polyamine depletion may have additional effects on immune responses, which is an area of active investigation in our laboratory.
The use of the pharmacologic agent, DFMO, to inhibit ODC has potential limitations compared to a genetic approach. We have used DFMO in our studies because homozygous deletion of ODC in mice is lethal.28 ODC+/− mice do survive to adulthood, and reduced lymphomagenesis when crossed to a c-Myc-overexpressing mouse has been demonstrated; these results also occurred with chronic oral DFMO treatment,28 further validating the approach we used in the current study. Moreover, we have shown that DFMO effectively reduced gastric polyamine levels in H. pylori-infected mice to the same levels as in uninfected mice. We have obtained the ODC+/− mice to determine the effect of ODC heterozygosity on gastric polyamine levels and the immunopathogenesis of H. pylori infection.
Deleterious effects of DFMO have been reported in acute gastric injury models,45 and DFMO impairs wound repair of intestinal epithelial cells in vitro.46 However, our data demonstrate a benefit of decreased H. pylori colonization that was accompanied by a decrease in gastric inflammation, and DFMO had no effect on histology in uninfected mice. There is substantial interest in DFMO as a chemoprevention agent in colon carcinogenesis, since it inhibits intestinal tumor growth in mouse models,31 and was effective in prevention of recurrent colonic adenomas in combination with sulindac in a human trial.47 Ultimately, DFMO could prove to be a useful adjunctive treatment for H. pylori, especially considering problems of antibiotic resistance and issues of reinfection after antibiotic treatment in areas of very high prevalence.
Supplementary Material
Acknowledgments
Supported by National Institutes of Health grants R01DK053620 and R01AT004821 (to K.T.W.), P01CA028842 (to K.T.W), P01CA116087 (K.T.W), F31GM083500 (to N.D.L), R01CA051085 and R01CA098454 (to R.A.C), T32CA009592 (N.D.L.), T32DK007673 (D.P.B.), the Flow Cytometry Core of the Vanderbilt University Digestive Disease Research Center grant (P30DK058404), a Merit Review Grant from the Office of Medical Research, Department of Veterans Affairs (to K.T.W.), and the Philippe Foundation (T.S. and A.P.G.).
Abbreviations
- CAT
cationic amino acid transporter
- DFMO
α-difluoromethylornithine
- FITC
fluorescein isothiocyanate
- iNOS
inducible nitric oxide synthase
- HPL
H. pylori lysate
- L-Arg
L-arginine
- L-Lys
L-lysine
- MOI
multiplicity of infection
- NO
nitric oxide
- ODC
ornithine decarboxylase
- RT-PCR
reverse transcription polymerase chain reaction
- Scr
scrambled
- siRNA
small interfering RNA
- Spm
spermine
Footnotes
Conflicts of Interest: None
Writing Assistance: None
Author contributions:
Rupesh Chaturvedi: study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, statistical analysis
Mohammad Asim: acquisition of data; technical support
Svea Hoge: acquisition of data
Nuruddeen D. Lewis: acquisition of data
Kshipra Singh: technical support
Daniel P. Barry: technical support
Thibaut de Sablet: technical support
M. Blanca Piazuelo: acquisition of data
Aditya R. Sarvaria: acquisition of data
Yulan Cheng: acquisition of data
Ellen I. Closs: material support, critical revision of manuscript for intellectual content
Robert A. Casero, Jr.: material support, acquisition of data, critical revision of manuscript for intellectual content
Alain P. Gobert: acquisition of data, critical revision of manuscript for intellectual content
Keith T. Wilson: study concept and design, analysis and interpretation of data, drafting of the manuscript, critical revision of manuscript for important intellectual content, statistical analysis, obtained funding, study supervision
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fox JG, Wang TC. Inflammation, atrophy, and gastric cancer. J Clin Invest. 2007;117:60–69. doi: 10.1172/JCI30111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Varanasi RV, Fantry GT, Wilson KT. Decreased prevalence of Helicobacter pylori infection in gastroesophageal reflux disease. Helicobacter. 1998;3:188–194. doi: 10.1046/j.1523-5378.1998.08001.x. [DOI] [PubMed] [Google Scholar]
- 3.Mai UE, Perez-Perez GI, Allen JB, et al. Surface proteins from Helicobacter pylori exhibit chemotactic activity for human leukocytes and are present in gastric mucosa. J Exp Med. 1992;175:517–525. doi: 10.1084/jem.175.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilson KT, Ramanujam KS, Mobley HLT, et al. Helicobacter pylori stimulates inducible nitric oxide synthase expression and activity in a murine macrophage cell line. Gastroenterology. 1996;111:1524–1533. doi: 10.1016/s0016-5085(96)70014-8. [DOI] [PubMed] [Google Scholar]
- 5.Gobert AP, Cheng Y, Wang JY, et al. Helicobacter pylori induces macrophage apoptosis by activation of arginase II. J Immunol. 2002;168:4692–4700. doi: 10.4049/jimmunol.168.9.4692. [DOI] [PubMed] [Google Scholar]
- 6.Gobert AP, McGee DJ, Akhtar M, et al. Helicobacter pylori arginase inhibits nitric oxide production by eukaryotic cells: a strategy for bacterial survival. Proc Natl Acad Sci U S A. 2001;98:13844–13849. doi: 10.1073/pnas.241443798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meyer F, Ramanujam KS, Gobert AP, et al. Cutting edge: cyclooxygenase-2 activation suppresses Th1 polarization in response to Helicobacter pylori. J Immunol. 2003;171:3913–3917. doi: 10.4049/jimmunol.171.8.3913. [DOI] [PubMed] [Google Scholar]
- 8.Wilson KT, Crabtree JE. Immunology of Helicobacter pylori: insights into the failure of the immune response and perspectives on vaccine studies. Gastroenterology. 2007;133:288–308. doi: 10.1053/j.gastro.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 9.Necchi V, Candusso ME, Tava F, et al. Intracellular, intercellular, and stromal invasion of gastric mucosa, preneoplastic lesions, and cancer by Helicobacter pylori. Gastroenterology. 2007;132:1009–1023. doi: 10.1053/j.gastro.2007.01.049. [DOI] [PubMed] [Google Scholar]
- 10.Forstermann U, Closs EI, Pollock JS, et al. Nitric oxide synthase isozymes. Characterization, purification, molecular cloning, and functions. Hypertension. 1994;23:1121–1131. doi: 10.1161/01.hyp.23.6.1121. [DOI] [PubMed] [Google Scholar]
- 11.Stuehr DJ, Cho HJ, Kwon NS, et al. Purification and characterization of the cytokine-induced macrophage nitric oxide synthase: an FAD- and FMN-containing flavoprotein. Proc Natl Acad Sci U S A. 1991;88:7773–7777. doi: 10.1073/pnas.88.17.7773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kakuda DK, Sweet MJ, Mac Leod CL, et al. CAT2-mediated L-arginine transport and nitric oxide production in activated macrophages. Biochem J. 1999;340 (Pt 2):549–553. [PMC free article] [PubMed] [Google Scholar]
- 13.Nicholson B, Manner CK, Kleeman J, et al. Sustained nitric oxide production in macrophages requires the arginine transporter CAT2. J Biol Chem. 2001;276:15881–15885. doi: 10.1074/jbc.M010030200. [DOI] [PubMed] [Google Scholar]
- 14.Yeramian A, Martin L, Serrat N, et al. Arginine transport via cationic amino acid transporter 2 plays a critical regulatory role in classical or alternative activation of macrophages. J Immunol. 2006;176:5918–5924. doi: 10.4049/jimmunol.176.10.5918. [DOI] [PubMed] [Google Scholar]
- 15.Bussiere FI, Chaturvedi R, Cheng Y, et al. Spermine causes loss of innate immune response to Helicobacter pylori by inhibition of inducible nitric-oxide synthase translation. J Biol Chem. 2005;280:2409–2412. doi: 10.1074/jbc.C400498200. [DOI] [PubMed] [Google Scholar]
- 16.Chaturvedi R, Asim M, Lewis ND, et al. L-Arginine availability regulates inducible nitric oxide synthase-dependent host defense against Helicobacter pylori. Infect Immun. 2007;75:4305–4315. doi: 10.1128/IAI.00578-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fu S, Ramanujam KS, Wong A, et al. Increased expression and cellular localization of inducible nitric oxide synthase and cyclooxygenase 2 in Helicobacter pylori gastritis. Gastroenterology. 1999;116:1319–1329. doi: 10.1016/s0016-5085(99)70496-8. [DOI] [PubMed] [Google Scholar]
- 18.Mannick EE, Bravo LE, Zarama G, et al. Inducible nitric oxide synthase, nitrotyrosine, and apoptosis in Helicobacter pylori gastritis: effect of antibiotics and antioxidants. Cancer Res. 1996;56:3238–3243. [PubMed] [Google Scholar]
- 19.Eaton KA, Mefford M, Thevenot T. The role of T cell subsets and cytokines in the pathogenesis of Helicobacter pylori gastritis in mice. J Immunol. 2001;166:7456–7461. doi: 10.4049/jimmunol.166.12.7456. [DOI] [PubMed] [Google Scholar]
- 20.DeLyria ES, Redline RW, Blanchard TG. Vaccination of mice against H pylori induces a strong Th-17 response and immunity that is neutrophil dependent. Gastroenterology. 2009;136:247–256. doi: 10.1053/j.gastro.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chaturvedi R, Cheng Y, Asim M, et al. Induction of polyamine oxidase 1 by Helicobacter pylori causes macrophage apoptosis by hydrogen peroxide release and mitochondrial membrane depolarization. J Biol Chem. 2004;279:40161–40173. doi: 10.1074/jbc.M401370200. [DOI] [PubMed] [Google Scholar]
- 22.Cheng Y, Chaturvedi R, Asim M, et al. Helicobacter pylori-induced macrophage apoptosis requires activation of ornithine decarboxylase by c-Myc. J Biol Chem. 2005;280:22492–22496. doi: 10.1074/jbc.C500122200. [DOI] [PubMed] [Google Scholar]
- 23.Gobert AP, Mersey BD, Cheng Y, et al. Cutting Edge: Urease release by Helicobacter pylori stimulates macrophage inducible nitric oxide synthase. J Immunol. 2002;168:6002–6006. doi: 10.4049/jimmunol.168.12.6002. [DOI] [PubMed] [Google Scholar]
- 24.Verrey F, Closs EI, Wagner CA, et al. CATs and HATs: the SLC7 family of amino acid transporters. Pflugers Arch. 2004;447:532–542. doi: 10.1007/s00424-003-1086-z. [DOI] [PubMed] [Google Scholar]
- 25.Closs EI, Basha FZ, Habermeier A, et al. Interference of L-arginine analogues with L-arginine transport mediated by the y+ carrier hCAT-2B. Nitric Oxide. 1997;1:65–73. doi: 10.1006/niox.1996.0106. [DOI] [PubMed] [Google Scholar]
- 26.Bussiere FI, Chaturvedi R, Asim M, et al. Low multiplicity of infection of Helicobacter pylori suppresses apoptosis of B lymphocytes. Cancer Res. 2006;66:6834–6842. doi: 10.1158/0008-5472.CAN-05-4197. [DOI] [PubMed] [Google Scholar]
- 27.Gobert AP, Cheng Y, Akhtar M, et al. Protective role of arginase in a mouse model of colitis. J Immunol. 2004;173:2109–2117. doi: 10.4049/jimmunol.173.3.2109. [DOI] [PubMed] [Google Scholar]
- 28.Nilsson JA, Keller UB, Baudino TA, et al. Targeting ornithine decarboxylase in Myc-induced lymphomagenesis prevents tumor formation. Cancer Cell. 2005;7:433–444. doi: 10.1016/j.ccr.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 29.Asim M, Chaturvedi R, Hoge S, et al. Helicobacter pylori induces ERK-dependent formation of a phospho-C-FOS/C-JUN AP-1 complex that causes apoptosis in macrophages. J Biol Chem. 2010 doi: 10.1074/jbc.M110.116988. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beaumier L, Castillo L, Ajami AM, et al. Urea cycle intermediate kinetics and nitrate excretion at normal and "therapeutic" intakes of arginine in humans. Am J Physiol. 1995;269:E884–896. doi: 10.1152/ajpendo.1995.269.5.E884. [DOI] [PubMed] [Google Scholar]
- 31.Yerushalmi HF, Besselsen DG, Ignatenko NA, et al. Role of polyamines in arginine-dependent colon carcinogenesis in Apc(Min) (/+) mice. Mol Carcinog. 2006;45:764–773. doi: 10.1002/mc.20246. [DOI] [PubMed] [Google Scholar]
- 32.Scott Algood HM, Gallo-Romero J, Wilson KT, et al. Host response to Helicobacter pylori infection before initiation of the adaptive immune response. FEMS Immunol Med Microbiol. 2007;51:577–586. doi: 10.1111/j.1574-695X.2007.00338.x. [DOI] [PubMed] [Google Scholar]
- 33.Rad R, Brenner L, Bauer S, et al. CD25+/Foxp3+ T cells regulate gastric inflammation and Helicobacter pylori colonization in vivo. Gastroenterology. 2006;131:525–537. doi: 10.1053/j.gastro.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 34.Peteroy-Kelly M, Venketaraman V, Connell ND. Effects of Mycobacterium bovis BCG infection on regulation of L-arginine uptake and synthesis of reactive nitrogen intermediates in J774.1 murine macrophages. Infect Immun. 2001;69:5823–5831. doi: 10.1128/IAI.69.9.5823-5831.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mossner J, Hammermann R, Racke K. Concomitant down-regulation of L-arginine transport and nitric oxide (NO) synthesis in rat alveolar macrophages by the polyamine spermine. Pulm Pharmacol Ther. 2001;14:297–305. doi: 10.1006/pupt.2001.0297. [DOI] [PubMed] [Google Scholar]
- 36.Lopatin AN, Makhina EN, Nichols CG. Potassium channel block by cytoplasmic polyamines as the mechanism of intrinsic rectification. Nature. 1994;372:366–369. doi: 10.1038/372366a0. [DOI] [PubMed] [Google Scholar]
- 37.Zhang M, Borovikova LV, Wang H, et al. Spermine inhibition of monocyte activation and inflammation. Mol Med. 1999;5:595–605. [PMC free article] [PubMed] [Google Scholar]
- 38.Moruzzi MS, Marverti G, Piccinini G, et al. The effect of spermine on calcium requirement for protein kinase C association with phospholipid vesicles. Int J Biochem Cell Biol. 1995;27:783–788. doi: 10.1016/1357-2725(95)00054-s. [DOI] [PubMed] [Google Scholar]
- 39.Endo M, Oyadomari S, Terasaki Y, et al. Induction of arginase I and II in bleomycin-induced fibrosis of mouse lung. Am J Physiol Lung Cell Mol Physiol. 2003;285:L313–321. doi: 10.1152/ajplung.00434.2002. [DOI] [PubMed] [Google Scholar]
- 40.Konturek PC, Rembiasz K, Konturek SJ, et al. Gene expression of ornithine decarboxylase, cyclooxygenase-2, and gastrin in atrophic gastric mucosa infected with Helicobacter pylori before and after eradication therapy. Dig Dis Sci. 2003;48:36–46. doi: 10.1023/a:1021774029089. [DOI] [PubMed] [Google Scholar]
- 41.Linsalata M, Russo F, Notarnicola M, et al. Polyamine profile in human gastric mucosa infected by Helicobacter pylori. Ital J Gastroenterol Hepatol. 1998;30:484–489. [PubMed] [Google Scholar]
- 42.Russo F, Linsalata M, Giorgio I, et al. Polyamine levels and ODC activity in intestinal-type and diffuse-type gastric carcinoma. Dig Dis Sci. 1997;42:576–579. doi: 10.1023/a:1018803311122. [DOI] [PubMed] [Google Scholar]
- 43.Miyazawa M, Suzuki H, Masaoka T, et al. Suppressed apoptosis in the inflamed gastric mucosa of Helicobacter pylori-colonized iNOS-knockout mice. Free Radic Biol Med. 2003;34:1621–1630. doi: 10.1016/s0891-5849(03)00218-1. [DOI] [PubMed] [Google Scholar]
- 44.Lee MJ, Huang CY, Sun YJ, et al. Cloning and characterization of spermidine synthase and its implication in polyamine biosynthesis in Helicobacter pylori strain 26695. Protein Expr Purif. 2005;43:140–148. doi: 10.1016/j.pep.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 45.Wang JY, Johnson LR. Luminal polyamines stimulate repair of gastric mucosal stress ulcers. Am J Physiol. 1990;259:G584–592. doi: 10.1152/ajpgi.1990.259.4.G584. [DOI] [PubMed] [Google Scholar]
- 46.Rao JN, Li J, Li L, et al. Differentiated intestinal epithelial cells exhibit increased migration through polyamines and myosin II. Am J Physiol. 1999;277:G1149–1158. doi: 10.1152/ajpgi.1999.277.6.G1149. [DOI] [PubMed] [Google Scholar]
- 47.Meyskens FL, McLaren CE, Pelot D, et al. Difluoromethylornithine plus sulindac for the prevention of sporadic colorectal adenomas: a randomized placebo-controlled, double-blind trial. Cancer Prev Res. 2008;1:32–38. doi: 10.1158/1940-6207.CAPR-08-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.