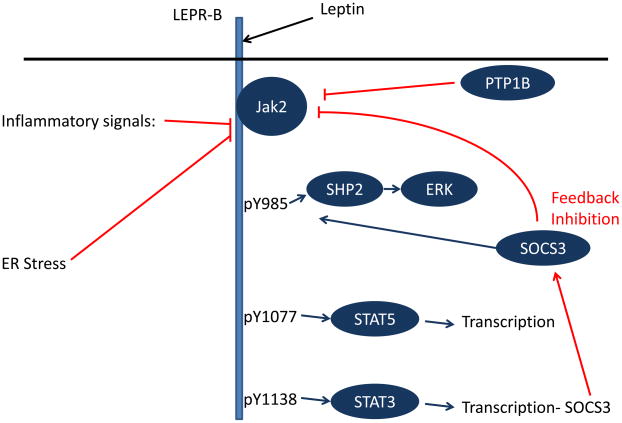
Figure 1.
Schematic diagram of LepRb signaling and mechanisms of cellular leptin resistance. Leptin binding promotes the activation of LEPR-B-associated Jak2, which phosphorylates three tyrosine residues on the intracellular tail of LEPR-B. Each of these phosphorylated residues recruits a unique set of downstream signaling molecules. Phosphorylated Tyr985 (pY985) recruits SHP2 (which participates in ERK activation) and SOCS3 (an inhibitor of LEPR-B signaling). pY1077 recruits the transcription factor STAT5, while pY1138 recruits STAT3. A variety of processes contribute to the attenuation of LEPR-B signaling (red lines), including the feedback inhibition that occurs by STAT3-promoted SOCS3 accumulation. PTP1B, ER stress, and inflammatory signals may also participate in the inhibition of LEPR-B signaling in obesity.