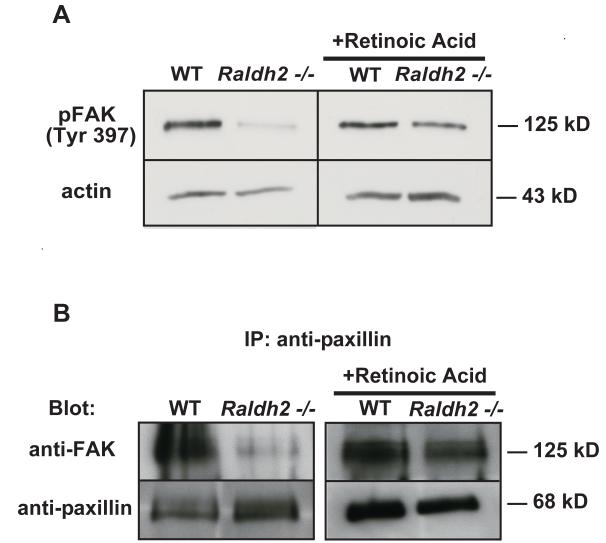
Figure 6. FAK activation and association with paxillin are diminished in Raldh2−/− mutants.
(A) Representative Western blots of phosphorylated FAK (Tyr397) in untreated WT and Raldh2−/− yolk sacs and in RA-treated (in vivo) WT and Raldh2−/− yolk sacs at 9.5 dpc demonstrate lower levels of FAK phosphorylation (Tyr397) in Raldh2−/− mutants compared to WT which is increased back to WT levels after in vivo rescue with RA. Actin was used as the loading control. (B) Paxillin was immunoprecipitated from protein lysates from 9.5 dpc WT and Raldh2−/− yolk sacs and representative Western blots show levels of FAK and paxillin. Complex formation between FAK and paxillin occurs in WT yolk sacs during normal vascular remodeling (B, left panel). In contrast, the association between FAK and paxillin was diminished in Raldh2−/− yolk sacs that fail to undergo vascular remodeling (B, left panel). However, after in vivo RA-rescue of yolk sac vascular remodeling in Raldh2−/− mutants, complex formation between FAK and paxillin was observed at a level similar to that detected in WT yolk sacs (B, right panel).