Abstract
NSC-741909 (1-[(4-chlorophenyl)methyl]-1H-Indole-3-methanol) is a novel anticancer agent that is highly active against several NCI-60 cancer cell lines. This agent induces sustained activation of mitogen-activated protein kinases (MAPK), including JNK and p38 MAP kinases. However, the mechanisms of its selective anti-tumor activity in some cancer cell lines remain unknown. We tested the combined effects of NSC-741909 and several kinase inhibitors that target the Raf/MEK/ERK1/2 or PI3K/AKT pathways in two sensitive lung cancer cells. We found that PD98059 (2'-amino-3'-methoxyflavone), a flavone derivative and a selective MEK inhibitor, can dramatically block the cell killing effect of NSC-741909. To determine whether this inhibitory effect is associated with MEK inhibition or other mechanisms, we evaluated the effects of other MEK inhibitors with different chemical structures and flavone derivatives that do not have an effect on MEK. We found that several flavonoids can markedly block NSC-741909-induced apoptosis and JNK activation in a time-dependent manner, regardless of whether they inhibit MEK or not. In contrast, NSC-741909-induced JNK activation and apoptosis were not blocked by other MEK-specific inhibitors U0126 and CI-1040. Our results also showed that NSC-741909 induced a dramatic increase of reactive oxygen species in sensitive cells and that flavonoids effectively blocked the NSC-741909-induced reactive oxygen species production which are associated with flavonoids’ antagonistic effects on NSC-741909-induced JNK activation and apoptosis. Those results demonstrated that flavonoids mediated antagonist effect is through scavenging of reactive oxygen species. Our results may have implication on the design of clinical evaluation of antitumor activity of NSC-741909 or its analogues.
Keywords: NSC-741909, flavonoids, antitumor, JNK, reactive oxygen species
1.Introduction
Mutations of three oncogenic Ras genes, especially the K-Ras gene, are found in approximately 90% of pancreatic cancers, 50% of colon cancers, and 35% of lung adenocarcinomas and are known to be associated with resistance to chemotherapy and radiotherapy (Bernhard et al.2000;Guerrero et al.2000;Nemunaitis et al.1997). Mouse strains carrying K-ras alleles, which can be activated by spontaneous recombination events, were highly predisposed to developing various tumor types, predominantly early-onset lung cancer (Johnson et al.2001). Moreover, withdrawal of doxycycline-inducible oncogenic H-ras or K-ras causes apoptosis in tumor cells and regression of tumors in transgenic mice (Chin et al.1999;Fisher et al.2001). Therefore, Ras gene mutations play an important role in tumorigenisis and the maintenance of malignant phenotypes.
As important mediators of signals from growth factor, cytokine, or mitogen receptors, Ras proteins are the common upstream molecules of several signaling pathways, including the Raf/MEK/ERK, PI3K/Akt, and RALGDS/Ral pathways (Downward, 2003;Schubbert et al.2007). Biochemical studies have revealed that guanosine triphosphate (GTP)-bound Ras binds to Raf proteins and recruits Raf to the plasma membrane, leading to activation of the Raf/MEK/ERK cascade (Leevers et al.1994;Marais et al.1995). Similarly, Ras directly interacts with the catalytic subunit of phosphatidylinositol-3-OH kinase (PI3K) in a GTP-dependent manner and activates PI3K (Pacold et al.2000). PI3Ks phosphorylate the integral membrane phosphotidylinositols at the 3’ position to generate a short-lived second messenger product, such as phosphatidylinositol (3,4,5)-phosphate (PIP3) (Vivanco and Sawyers 2002). Membrane-associated PIP3 regulates the activity of a variety of signaling molecules, including the Akt/PKB serine/threonine kinases. The Raf/MEK/ERK and the PI3K/AKT pathways play crucial roles in regulating proliferation, differentiation, and apoptosis in tumor cells (Merighi et al.2006), and each component of these pathways has been extensively explored as targets for anticancer therapy (Downward, 2003).
We recently identified a small compound (oncrasin-1) that can selectively kill cells harboring K-Ras mutations (Guo et al.2008). Upon testing various oncrasin-1 analogues, we identified a small compound, NSC-741909, that is highly active against and has a unique anticancer spectrum in several NCI-60 cancer cell lines, suggesting that it could be a novel anticancer agent. We also found that this agent can induce sustained JNK activation and suppress the expression of MAPK phosphatase 1 (MKP1). However, the mechanisms by which NSC-741909 induces apoptosis selectively in some cancer cell lines remain to be determined.
To investigate the molecular mechanisms underlying NSC-741909-mediated antitumor activity, we tested the effect of combining NSC-741909 with several kinase inhibitors targeting the Raf/MEK/ERK1/2 or PI3K/AKT pathways. We found that PD98059 (2'-amino-3'-methoxyflavone), a flavone derivative and a selective MEK inhibitor (Dudley et al.1995), blocked the cell killing effect of NSC-741909. However, NSC-741909-induced apoptosis was not blocked by U0126 and CI-1040, two other MEK inhibitors, but was blocked by the other flavone derivatives, such as genistein and apigenin, which do not inhibit MEK, suggesting that PD98059's blocking effect is independent of MEK/ERK pathway. Our study also revealed that NSC-741909 induced a dramatic increase of reactive oxygen species (ROS) in sensitive cells and that flavonoids mediated antagonist effect is through scavenging of the NSC-741909-induced reactive oxygen species production.
2. Material and Methods
2.1 Cultured cells
The human non–small cell lung carcinoma H460 ,H157, H322 and normal human fibroblast (NHFB) cell lines were routinely propagated in a monolayer culture in RPMI 1640 medium supplemented with 10% heat-inactivated fetal calf serum, 100 units/ml penicillin, and 100 mg/ml streptomycin. All cells were maintained in the presence of 5% CO2 at 37°C.
2.2 Cytotoxicity studies
We determined cell viability using the sulforhodamine B (SRB) assay. Cells (4 × 103 in 100 μl of culture medium/well) were seeded in 96-well, flat-bottomed plates and treated the next day with the drugs at the indicated concentrations. After 48 h, cells were washed once with phosphate-buffered saline (PBS), and cell viability was determined on SRB assay, as described previously (Guo et al.2009;Pauwels et al.2003). Briefly, after fixation of the adherent cells with trichloroacetic acid in a 96-well microplate, the cells were stained with SRB, and the optical density was determined at 570 nm to reflect the number of stained cells. We determined the relative cell viability by normalizing the cells to the DMSO-treated control cells, which was set at 1. Each experiment was performed in quadruplicate and repeated at least three times.
2.3 Flow cytometry assays
For apoptosis analysis, cells were treated with NSC-741909 (1 μM) with or without kinase inhibitors for the indicated times as described in the results. Briefly, after treatment, cells were harvested with trypsin, washed once with fresh medium-containing serum, and then washed twice with PBS. Cells were fixed with 75% ethanol overnight. Next, the cell pellets were harvested and re-suspended with propidium iodide (BD San Jose, CA) for 15 min in a dark place at room temperature. For reactive oxygen species analysis, the cell-permeable nonfluorescent compound H2DCF-DA was used for measuring intracellular reactive oxygen species. H2DCF-DA was dissolved in dimethylsulfoxide and diluted with phosphate-buffered saline (PBS) to a final concentration of 5 μmol/L. Cells were seeded at a density of 2.5 × 105 cells/well in six-well plates and allowed to grow overnight. The cells were treated either with 1 μM NSC-741909 alone for 6 h or with 10 μM PD98059, genistein ,apigenin and U0126 pretreatment for half an hour before NSC-741909 was added. Subsequently, 5 μmol/L H2DCF-DA was added, and cells were incubated for 40 min at 37°C. Cells were then returned to a prewarmed growth medium and incubated for 10 min at 37°C. Cells were harvested with trypsin and washed once with PBS, and the fluorescence intensity was determined using flow cytometry, with excitation and emission settings of 488 and 530 nm, respectively. The mean fluorescence peak was analyzed from the gated cell population of 10,000 cells. All experiments were performed three times. The flow cytometry assays were performed at the Flow Cytometry and Cellular Imaging Facility at The University of Texas M. D. Anderson Cancer Center.
2.4 Western blot analysis
To prepare whole-cell extracts, cells were washed twice in cold PBS, collected, and then lysed in lysis buffer (62.5 mM Tris [pH 6.8], 2% sodium dodecyl sulfate, and 10% glycerol) containing 1× proteinase-inhibitor cocktail (Roche, Indianapolis, IN). The lysates were spun at 14,000 × g in a microcentrifuge at 4°C for 10 min. The supernatants were then used as the whole-cell extracts. Equal amounts (50–100 μg) of proteins were used for immunoblotting. Proteins were subjected to electrophoresis under reducing conditions on 8–12.5% (w/v) polyacrylamide gels and then electrophoretically transferred to nitrocellulose transfer membranes (Amersham, Piscataway, NJ). The membranes were incubated with the primary antibody followed by the peroxidase-linked secondary antibody. An chemiluminescence Western blotting system (GE Healthcare, Chalfont St. Giles, United Kingdom) was used to detect the secondary probes.
2.5 Antibodies and reagents
We purchased antibodies against JNK, phospho-JNK (pThr183/pTyr185), ERK1/2 and phospho-ERK1/2 ( pThr202/tyr204), phospho-pAkt (pSer473), phosphor-p38, cleaved PARP and phospho-c-Jun (pSer63) from Cell Signaling Technology (Danvers, MA); caspase 8 from BD Biosceinces ( San Jose,CA) and β-actin from Sigma-Aldrich (St. Louis, MO). NSC-741909 was synthesized by Zhejiang Yuancheng (MST Inc., Hangzhou, China). 2’,7’-Dichlorofluorescein diacetate (H2DCF-DA) was purchased from Invitrogen Molecular Probes (Carlsbad, CA, USA).
2.6 Statistical analysis
Statistical differences between treatment groups were assessed by analysis of variance (ANOVA) using StatSoft software (Tulsa, OK, USA). P values of < 0.05 were regarded as significant.
3. Results
3.1 PD98059 blocks the cell killing effect of NSC-741909
NSC-741909 is effective against several human cancer cell lines derived from lung, colon, ovary, kidney and breast cancers, including those with or without K-Ras mutations. To delineate NSC-741909's molecular mechanisms of action, we investigated the roles of several key molecules in signaling pathways that are critical in maintenance of malignant phenotypes and might be associated with NSC-741909-induced apoptosis. For this purpose, we examined the effect of NSC-741909 in the presence or absence of kinase inhibitors specific for MEK, PI3K, Akt, and p38, most of which are critically involved in downstream Ras signaling.
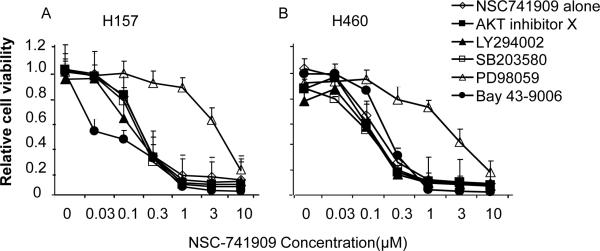
The sensitive lung cancer cell lines H157 and H460 were treated with 0.03-10 μM NSC-741909 alone or in combination with 10 μM Akt inhibitor X, an Akt-specific inhibitor (Thimmaiah et al.2005); LY294002, a PI3K inhibitor (Shoba et al.2001); SB203580, a p38 MAPK inhibitor (Badger et al.1998); Bay-43-9006, a RAF kinase inhibitor (Panka et al.2006); or PD98059, a MEK-specific inhibitor. We determined cell viability 48 h after treatment. We found that, at the concentrations used, those inhibitors alone had minimal impact on cell viabilities and that the presence of Akt inhibitor X, LY294002, Bay-43-9006 and SB203580 did not affect the cell killing effect of NSC-741909 (Fig. 1). However, the presence of PD98059 dramatically reversed the cell killing effect of NSC-741909 in both cell lines. When the H460 and H157 cells were treated with NSC-741909 alone, the IC50 values of NSC-741909 were 0.19 μM and 0.1 μM, respectively; however, the IC50 values increased to 3.16 μM and 5 μM, respectively, when the cells were treated with NSC-741909 and PD98059, demonstrating that PD98059 has antagonist effect on NSC-741909-induced apoptosis.
Fig. 1.
Antagonistic effect of PD98059 on NSC-741909-induced cell killing in H460 and H157 cells. H157 (A) and H460 (B) cells were treated with NSC-741909 alone or with 10 μM of AKT inhibitor X, LY294002, SB203580, or PD98059, or 1μM of Bay-439006 0.5 h before NSC-741909 treatment. Then, the dose response to NSC-741909 was determined after 48 h. An SRB assay was performed to determine the cell viability, and the viability of cells treated with DMSO was set at 1. The effects of the inhibitors alone were presented at the data points when NSC-741909 was 0.
3.2 Flavonoids but not MEK inhibitors block the cell killing effect of NSC-741909
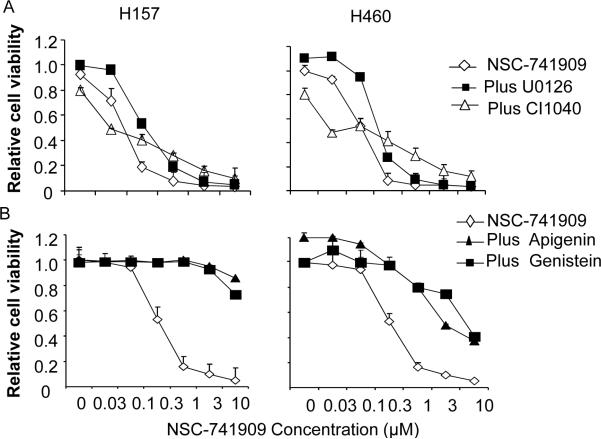
PD98059 is commonly used as a tool for dissecting the contributions of distal members of the MAPK cascade to the biological processes (Cook et al.1997). In addition to inhibiting the MEK pathway, PD98059 can also induce other biological functions. To test whether the PD98059-mediated blocking effect is MEK dependent or independent, we evaluated the effects of two other MEK inhibitors, U0126 (Favata et al.1998) and CI-1040 (Solit et al.2006), which have different chemical structures than PD98059. Treatment of the H157 and H460 cells lines with U0126 and CI-1040 at doses that were sufficient to block ERK phosphorylaton did not induce the blocking effects seen with PD98059 (Fig. 2A), suggesting PD98059 mediated blocking effect is independent of MEK/ERK pathway.
Fig. 2.
Antagonistic effects of flavonoids genistein and apigenin. (A) H157 and H460 cells were treated with NSC-741909 alone or with 10 μM of U0126 or 1μM of CI1040 0.5 h before NSC-741909 treatment. Then, the dose response to NSC-741909 was determined after 48 h. (B) H157 and H460 cells were treated with NSC-741909 alone or with 10 μM genistein or apigenin 0.5 h before NSC-741909 treatment. Again, the dose response to NSC-741909 was determined after 48 h. The effects of the MEK inhibitors or flavonoids alone were presented at the data points when NSC-741909 was 0.
Because PD98059 is a flavonoid, we then evaluated whether other flavonoids might have effects similar to those of PD98059. For this purpose, we determined the dose response of NSC-741909 in the H157 and H460 cells in the presence or absence of 10 μM genistein or apigenin (see chemical structures on Supplemental S1). These two flavonoids reversed the cell killing effect of NSC-741909, which was similar to that observed with PD98059 (Fig. 2B), suggesting that the PD98059-induced blocking effect could be mediated by some biological function that is common for flavonoids.
3.3 Flavonoids inhibits NSC-741909-mediated MAPK activation
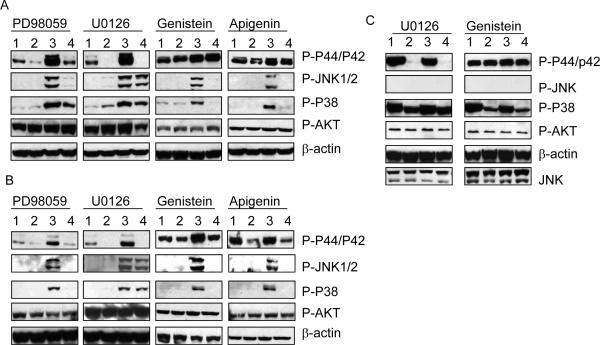
We performed Western blot analyses to investigate the mechanisms by which PD98059, genistein, and apigenin blocked NSC-741909-induced antitumor activity and to test the effect of those compounds on MAPKs, including ERK, p38, and JNK. H460 and H157 cell lines were treated with 1 μM NSC-741909 in the presence or absence of 10 μM PD98059, U0126, genistein, or apigenin. Cells were harvested 12 h after treatment, and the cell lysates were used for Western blot assay. Cells treated with dimethyl sulfoxide (DMSO) or PD98059, U0126, genistein, and apigenin alone were used as controls.
We found that in both cell lines, the levels of phosphorylated ERK, JNK, and p38 were dramatically increased after treatment with NSC-741909, whereas no obvious changes or only minor changes were observed for phosphorylated Akt. Both PD98059 and U0126 suppressed endogenous and NSC-741909-induced phosphorylation of ERK. PD98059, genistein, and apigenin suppressed NSC-741909-induced phosphorylation of JNK and suppressed NSC-741909-induced phosphorylation of p38, although PD98059 was less effective than genistein and apigenin in suppressing p38 phosphorylation (Fig. 3A and 3B). Although U0126 suppressed endogenous and NSC-741909-induced phosphorylation of ERK, U0126 had no effect on NSC-741909-induced phosphorylation of JNK and p38. In contrast, in NSC-741909 resistant H322 cell line there was little change in the phosphorylation levels of Akt, p38, p44/p42, and JNK after treatment with NSC-741909 (Fig. 3C). In H322 cells, genistein reduced endogenous phosphorylation of p38, whereas U0126 reduced endogenous phosphorylation of ERK. Because U0126 effectively suppressed endogenous and NSC-741909-induced phophsorylation of ERK but had no impact on NSC-741909-induced cell killing, we concluded that the antagonism of PD98059 on NSC-741909 was MEK independent. This conclusion is supported by the fact that the PD98059 analogues apigenin and genistein had a similar antagonistic effect but had no impact on endogenous and NSC-741909-induced phosphorylation of ERK.
Fig. 3.
Effects on MAP kinase activation. The H157 (A), H460 (B), and H322 (C) cells were treated with DMSO, NSC-741909 alone, or 10 μM of PD98059, U0126, genistein, or apigenin 0.5 h after NSC-741909 treatment. We performed Western blot analysis to detect the changes of K-RAS downstream signals. Lane 1: DMSO; Lane 2: corresponding inhibitors; Lane 3: NCS-741909; Lane 4: combination of NSC-741909 and inhibitors.
3.4 Flavonoid-induced antagonism is time dependent
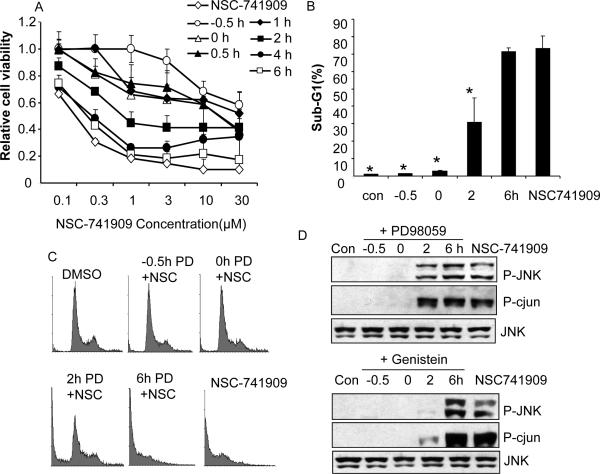
Next, we tested whether flavonoid-induced antagonism to NSC-741909 was time dependent. Therefore, we treated H460 cells with 10 μM PD98059 half an hour before (-0.5 h), at the same time as (0 h), or 0.5, 1, 2, 4, or 6 h after NSC-741909 was added to the cells. The dose response to NSC-741909 was then determined 48 h after NSC-741909 treatment. We found that adding PD98059 between -0.5 and 2 h after NSC-741909 treatment induced an antagonistic effect. No obvious antagonistic effects or only mild antagonistic effects were observed if PD98059 was added 4 h or later after NSC-741909 treatment (Fig. 4A). This result demonstrated that flavonoid-induced antagonism to NSC-741909 is time dependent.
Fig. 4.
Time-dependent antagonistic effects of PD98059. H460 cells were treated with NSC-741909 alone or NSC-741909 combined with 10 μM PD98059 given before (-0.5 h), at the same time as (0 h), or after (0.5, 1, 2, 4, or 6 h) NSC-741909 treatment. Cells were harvested after a total NSC-741909 treatment time of 48 h. Cells treated with DMSO were set up as the control. (A) Cell viability assay. (B) Sub-G1 percentage. Cells were harvested after a total NSC-741909 treatment time of 24 h. *P< 0.05, compared with cells treated with NSC-741909 alone. (C) FAC diagram. (D) PD98059 and genistein block NSC-741909-induced JNK activation. H460 cells were treated with NSC-741909 alone or NSC-741909 combined with 10 μM of PD98059 or 10 μM of genistein at different time points. Cells were harvested at the time when the total NSC-741909 treatment reached 12 h. Cells treated with DMSO were set up as the control. We performed Western blot analysis to detect the changes of JNK phosphorylation.
Flow cytometry assay confirmed this observation of NSC-741909-induced apoptosis 24h after NSC-741909 treatment. Adding PD98059 to cells -0.5 h before or at the same time as NSC-741909 treatment almost completely blocked NSC-741909-induced apoptosis, whereas adding PD98059 0.5 h, 2 h after NSC-741909 treatment blocked about 50% of NSC-741909-induced apoptosis. In contrast, there was no effect on NSC-741909-induced apoptosis if PD98059 was added 6 h after NSC-741909 treatment (Fig. 4B and C), confirming that the antagonistic effect of flavonoid compounds on NSC-741909 is time dependent.
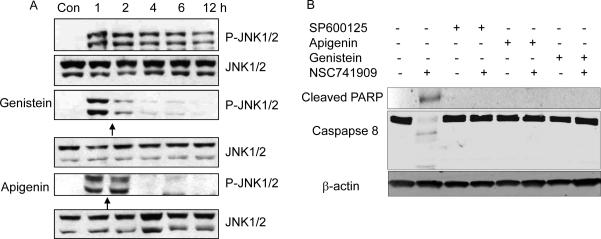
We also determined the effect of flavonoids on NSC-741909-induced JNK activation. We added 10 μM PD98059 and genistein to H460 cells at various times (0.5 h before, at the same time as, or 0.5, 2, 4, or 6 h after treatment with NSC-741909). Cells treated with DMSO or NSC-741909 alone was used as the controls. Cell lysates were harvested 12 h after NSC-741909 treatment and analyzed on Western blot. We found that adding PD98059 and genistein either half an hour earlier or at the same time as NSC-741909 completely blocked NSC-741909-induced phosphorylation of JNK and its substrate c-JUN. When added 2 h after NSC-741909, the blocking effect was partial for PD98059 and almost complete for genistein. However, 6 h after NSC-741909 was added, no blocking was observed for JNK and c-JUN phosphorylation (Fig. 4D).
3.5 Apigenin and Genistein can reverse NSC-741909 induced JNK activation and apoptosis
We have investigated whether flavonoids can reverse NSC-741909 induced JNK action. For this purpose, we treated H460 cells with 1 μM NSC-741909 for 1 h. Western blot analysis showed that this treatment was sufficient to activate JNK as evidenced by a dramatic increase of phosphor-JNK. 10 μM of genistein and 10 μM of apigenin were then added to cells, respectively. Cells were harvested overtime and levels of phosphor-JNK, were determined by Western blot analysis. The results showed that apigenin and genistein can diminish phosphor-JNK levels even if after NSC-741909 induced JNK activation had occurred (Fig. 5A). This result demonstrated that, at least at 1 h after NSC-741909 treatment, flavonoids were able to reverse NSC-741909 induced JNK activation.
Fig. 5.
Antagonistic effect of genistein and apigenin on NSC-741909-induced JNK activation and apoptosis. (A) Effect on JNK activation. After pretreatment with NSC-741909 for 1 hour, H460 cells were treated with 10 μM genistein or 10 μM apigenin for the times indicated in the text. Cells were harvested at the time when the total NSC-741909 treatment reached 12 h. Cells treated with DMSO were set as the control. We performed Western blot analysis to detect the changes of JNK phosphorylation. (B) Effect on apoptosis. H460 cells were pretreated with 10μM of genistein, apigenin or SP600125 (a JNK inhibitor) for half hour before addition of 1μM of NSC-741909. The cells were harvested 24 hours after NSC-741909 treatment. Levels of caspase 8 cleavage and cleaved PARP were determined.
We also determined whether presence of apigenin and genistein suppressed NSC-741909 induced apoptosis. To this end, H460 cells were pretreated with 10μM of genistein, apigenin or SP600125 (a JNK inhibitor) half hour before addition of 1μM of NSC-741909. The cells were harvested 24 hours after NSC-741909 treatment. Levels of caspase 8 cleavages and cleaved poly (ADP-ribose) polymerase (PARP) were determined by Western blot analysis (Fig. 5 B). The result showed that genistein and apigenin, like the JNK inhibitor SP600125, could block the NSC-741909 induced apoptosis.
3.6 Scavenging NSC-741909-induced reactive oxygen species by flavonoids
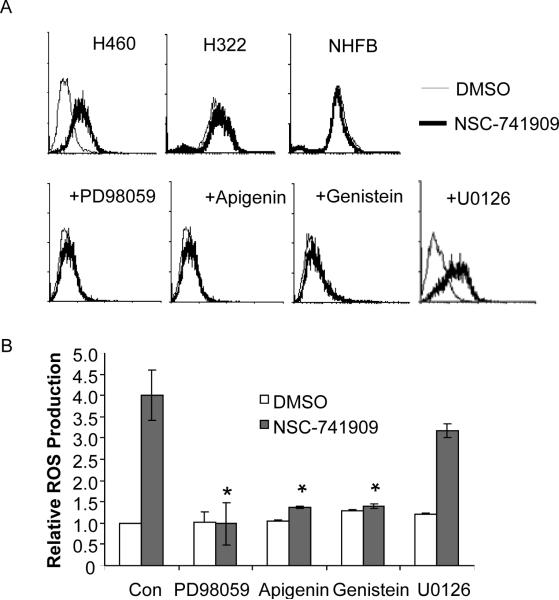
Our recent study showed that NSC-741909 induced sustained JNK activation by suppression of JNK dephosphorylation and the expression of MAPK phosphatase-1. It is well known that reactive oxygen species can inactivate protein phosphatases by oxidizing cysteines at the catalytic domain, which leads to increased phosphorylation and activation of many protein kinases, including MAP kinases (Kamata et al.2005;Knebel et al.1996). To test whether reactive oxygen species was involved in NSC-741909-mediated antitumor activity, we compared reactive oxygen species levels in normal human fibroblasts (NHFB), NSC-741909-sensitive (H460) and –resistant (H322) cancer cells, with or without NSC-741909 treatment. The result showed that treatment with NSC-741909 resulted in a dramatic increase of reactive oxygen species in H460 cells, but not in NHFB and H322 cells (Fig. 6A).
Fig. 6.
Flavonoids blocked NSC-741909-induced reactive oxygen species generation. The levels of reactive oxygen species were determined by flow cytometric analysis. Cells treated with DMSO were set as the control (Con). (A) A typical FAC diagram. Upper panel: H460, H322 and NHFB cells were treated with DMSO or 1μM NSC-7419091 for 6h. Lower panel: H460 cells were treated with 10 μM genistein, 10 μM PD98059, 10 μM apigenin or 10 μM U0126 for 0.5 h before adding 1 μM NSC-741909 for another 6 h. (B) Mean + S.D. of reactive oxygen species values determined in 3 independent times. *P< 0.05, compared with cells treated with NSC-741909 alone.
We then determined whether presence of flavonoids block NSC-741909-induced reactive oxygen species in H460 cells. For this purpose, H460 cells were treated with 1 μM of NSC-741909 alone for 6 h or pre-treated with 10 μM PD98059, U0126, genistein and apigenin, respectively, for half an hour before NSC-741909 was added. The generation of reactive oxygen species was then determined. The results showed that that all three flavoniods, but not U0126, significantly blocked the NSC-741909-induced reactive oxygen species generation (Fig. 6A and 6B). These results suggested that flavonoids can scavenge or block NSC-741909 mediated reactive oxygen species generation, thereby blocking its antitumor activity and reactive oxygen species mediated MAP kinase activation.
3.7 Association of Flavonoids-induced antagonism and their effects on reactive oxygen species scavenger and JNK activation
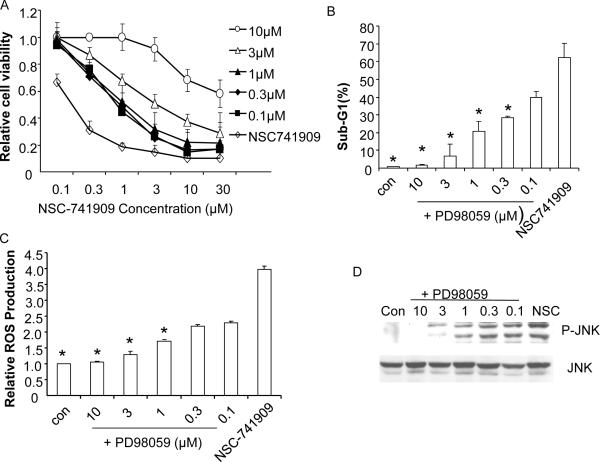
We further tested whether flavonoid-induced antagonism to NSC-741909 was associated with their effects on reactive oxygen species scavenging and suppression of JNK activation. To this end, H460 cells were treated with different doses of PD98059 half an hour before NSC-741909 was added to the cells. The cell viability to NSC-741909 was then determined 48 h after NSC-741909 treatment. We found that addition of PD98059 before NSC-741909 treatment induced an antagonistic effect in a dose dependent manner (Fig. 7A). We also determined dose response of PD98059-mediated antagonistic effects on NSC-741909-induced apoptosis, reactive oxygen species production and JNK activation. H460 cells were treated with different doses of PD98059 for half an hour and then with 1μM NSC-741909. Levels of apoptosis, reactive oxygen species and p-JNK were then determined. The results showed that PD98059-mediated antagonistic effects on NSC-741909-induced apoptosis, reactive oxygen species generation, and JNK activation were also dose dependent (Fig. 7B, C, and D). Similar dose-dependent antagonistic effects were observed when genistein was used to block NSC-741909-induced apoptosis, reactive oxygen species generation and JNK activation in H460 cells (data not shown). Together, those results demonstrated that flavonoids-mediated antagonistic effect on NSC-741909-induced apoptosis was associated with their effect on reactive oxygen species scavenging and JNK activation.
Fig. 7.
Association of Flavonoids-induced antagonism and their effects on reactive oxygen species scavenger and JNK activation. (A) Cell viability assay. H460 cells were treated with 0.1-10 μM PD98059 for 3 min before different doses of NSC-741909 were added to the cells. The cell viability was then determined 48 h after NSC-741909 treatment. Cells treated with DMSO were set up as the control. (B-D) H460 cells were treated with 0.1-10 μM PD98059 for half an hour and then with 1μM of NSC-741909. Cells treated with DMSO were set up as the control (con). Levels of apoptosis (B), reactive oxygen species (C) and p-JNK (D) were then determined. *P < 0.05, compared with cells treated with NSC-741909 alone.
4. Discussion
NSC-741909 is an analogue of oncrasin-1 which we identified as an anticancer agent through cell-based synthetic lethality screening (Guo et al. 2008). Testing of NCI-60 cell lines showed that NSC-741909 has a unique anticancer spectrum and is effective against a number of cancer cell lines derived from lung, colon, ovarian, kidney and breast cancers, suggesting its novel mechanisms (Wei et al.2009). Molecular characterization of NSC-741909 revealed that NSC-741909-induced activation of MAPK (including p38, JNK, and ERK) in sensitive cells and that suppression of MAPK dephosphorylation could be the primary cause of MAPK activation. The role of JNK activation in NSC-741909 induced apoptosis was further supported by fact that JNK inhibitors or dominant-negative JNK can effectively diminish NSC-741909-induced apoptosis. Because the prototype compound of NSC-741909, oncrasin-1, has been identified through library screening for selective cytotoxicity against oncogenic Ras-transformed cells, we tested whether Ras signaling pathways were involved in NSC-741909's induced cell context–dependent apoptosis. Effective antagonist effect of PD98059 (Dudley et al.1995), a well known MEK/ERK inhibitor, on NSC-741909 induced apoptosis led us to perform the studies presented here. Here, we demonstrated that PD98059 and other flavonoid analogues can effectively block NSC-741909-induced apoptosis. This blocking effect is associated with suppression of NSC-741909 induced JNK activation, but not with MEK/ERK inhibition.
PD98059 has been reported to exhibit antitumor cell proliferation and antitumor invasion and metastasis activity against various tumor cell species in vivo and in vitro (Sato et al.2002). However, attenuation of different apoptosis stimuli by PD98059 has also been previously reported, and some of PD98059's antiapoptotic effects may not be attributable to MEK/ERK inhibition. It was reported that PD98059 could attenuate hydrogen peroxide-induced cell death through inhibition of Jun N-terminal kinase in HT29 cells (Salh et al.2000). Kim reported that PD98059 could regulate GSH homeostasis independent of MEK/ERK inhibition (Kim et al.2006). Similar to PD98059, the flavonoids genistein and apigenin have been studied extensively for their antitumorigenic activities in various cancers, including prostate and breast cancers(Deorukhkar et al.2007). The reported mechanisms associated with these flavonoids’ antitumor action include induction of cell cycle arrest and apoptosis, inhibition of the action of transcription factors such as NF-κB, attenuation of the phosphorylation of epidermal growth factor receptor and MAPK (Wei et al.1996), inhibition of tyrosine kinases and activation of the intrinsic apoptosis pathway. In our study, we found that flavonoids, including PD98059, could block NSC-741909-induced JNK activation and apoptosis in cancer cells.
Flavonoids are polyphenolic compounds that are ubiquitous in nature and are categorized, according to chemical structure, into flavonols, flavones, flavanones, isoflavones, catechins, anthocyanidins and chalcones (Beecher, 2003). Over 4,000 flavonoids have been identified, many of which occur in fruits, vegetables and beverages (tea, coffee, beer, wine and fruit drinks) (Ficarra et al.2002). Genistein is one of isoflavones. Apigenin is a nonmutagenic bioflavonoid. Both of them are able to induce antagonist effect on NSC-741909 induced apoptosis without obvious effect on ERK phosphorylation, suggesting that flavonoids mediated antagonist effect is independent of MEK/ERK pathway. This antagonist effect is also not caused by chemical interactions between flavonoids and NSC-741909 because incubation flavonoids with NSC-741909 did not alter chemical profile of NSC-741909 (data not shown). Interestingly, all flavonoids that are able to block NSC-741909 induced apoptosis are able to JNK activation, supporting our recent finding that JNK activation is associated with NSC-741909 mediated antitumor activity (Wei et al.2009)
Several studies have shown that JNK functions as a proapoptotic kinase (Davis, 2000;Liu and Lin2005). The most direct evidence is that jnk1-/-/jnk2-/- mice were resistant to apoptosis induced by ultraviolet (UV) irradiation, anisomycin, and methylmethanesulfonate (MMS) (Tournier et al.2000;Weston and Davis2002). Since UV irradiation was unable to induce cytochrome C release or depolarization of the mitochondrial membrane potential in jnk1-/-/jnk2-/- mice, it was proposed that JNK is an intrinsic component of the mitochondrial-dependent death pathway during stress-induced apoptosis (Davis, 2000). In support of this conclusion, it was reported that in CHO cells, the constitutively active JNKK2- JNK1 fusion protein was sufficient to induce apoptosis by activating the intrinsic death pathway (Zheng et al.1999). Moreover, JNK activation was reported to promote flavopiridol/proteasome inhibitor-mediated lethality in leukemic cells that was also significantly diminished by agents and siRNA blocking JNK activation (Dai et al.2003). Further more, a JNK-dependent pathway is required for TNFalpha-induced apoptosis (Deng et al.2003). Activation of JNK induces caspase 8-independent cleavage of Bid at a distinct site, and translocation of cleaved Bid to mitochondria leads to preferential release of Smac/DIABLO, but not cytochrome c. TNF-alpha-induced apoptosis is suppressed by inhibition of the JNK pathway but promoted by its activation (Tang et al.2002). On the other hand, reactive oxygen species was found to be the critical components in TNF-alpha induced sustained JNK activation and apoptosis. Our recent study also revealed that NSC-741909 induced sustained JNK activation was largely caused by suppression of JNK dephosphorylation. Here we found that flavonoids can effectively scavenge NSC-741909 induced reactive oxygen species generation and JNK activation, suggesting that reactive oxygen species generation could be one of the mechanisms of NSC-741909 induced antitumor activity and that flavonoids’ antagonist effect was likely through scavenging of reactive oxygen species production.
Because flavonoids exist widely in various fruits, vegetables and beverages, the finding of the antagonist effects of flavonoids on NSC-741909 induced antitumor activity may have impact on preclinical and clinical assessment of antitumor activities of NSC-741909 and its analogues. Our data showed that, although flavonoids were able to reverse NSC-741909 induced JNK activation at 1 h after administration of NSC-741909, their antagonist effect was reduced to minimal at 4 – 6 h after treatment of NSC-741909. This information might have implication for design of in vivo experiments to evaluate antitumor activity of NSC-741909 or its analogues.
Supplementary Material
Acknowledgements
We would like to thank Alyson Todd for her editorial review of this manuscript. This work was supported by a National Cancer Institute grants R01 CA 092487 and RO1 CA 124951 (B. Fang) and Cancer Center Support Grant CA 16672), National Natural Science Foundation of China No.30973563 (to X Wei), Zhejiang Science Foundation (2008C14080) and INNOFUND of China (09C26213301228).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Badger AM, Cook MN, Lark MW, Newman-Tarr TM, Swift BA, Nelson AH, Barone FC, Kumar S. SB 203580 inhibits p38 mitogen-activated protein kinase, nitric oxide production, and inducible nitric oxide synthase in bovine cartilage-derived chondrocytes. J.Immunol. 1998;161:467–473. [PubMed] [Google Scholar]
- Beecher GR. Overview of dietary flavonoids: nomenclature, occurrence and intake. J.Nutr. 2003;133:3248S–3254S. doi: 10.1093/jn/133.10.3248S. [DOI] [PubMed] [Google Scholar]
- Bernhard EJ, Stanbridge EJ, Gupta S, Gupta AK, Soto D, Bakanauskas VJ, Cerniglia GJ, Muschel RJ, McKenna WG. Direct evidence for the contribution of activated N-ras and K-ras oncogenes to increased intrinsic radiation resistance in human tumor cell lines. Cancer Res. 2000;60:6597–6600. [PubMed] [Google Scholar]
- Chin L, Tam A, Pomerantz J, Wong M, Holash J, Bardeesy N, Shen Q, O'Hagan R, Pantginis J, Zhou H, Horner JW, Cordon-Cardo C, Yancopoulos GD, DePinho RA. Essential role for oncogenic Ras in tumour maintenance. Nature. 1999;400:468–472. doi: 10.1038/22788. [DOI] [PubMed] [Google Scholar]
- Cook SJ, Beltman J, Cadwallader KA, McMahon M, McCormick F. Regulation of mitogen-activated protein kinase phosphatase-1 expression by extracellular signal-related kinase-dependent and Ca2+-dependent signal pathways in Rat-1 cells. J.Biol.Chem. 1997;272:13309–13319. doi: 10.1074/jbc.272.20.13309. [DOI] [PubMed] [Google Scholar]
- Dai Y, Rahmani M, Grant S. Proteasome inhibitors potentiate leukemic cell apoptosis induced by the cyclin-dependent kinase inhibitor flavopiridol through a SAPK/JNK- and NF-kappaB-dependent process. Oncogene. 2003;22:7108–7122. doi: 10.1038/sj.onc.1206863. [DOI] [PubMed] [Google Scholar]
- Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- Deng Y, Ren X, Yang L, Lin Y, Wu X. A JNK-dependent pathway is required for TNFalpha-induced apoptosis. Cell. 2003;115:61–70. doi: 10.1016/s0092-8674(03)00757-8. [DOI] [PubMed] [Google Scholar]
- Deorukhkar A, Krishnan S, Sethi G, Aggarwal BB. Back to basics: how natural products can provide the basis for new therapeutics. Expert.Opin.Investig.Drugs. 2007;16:1753–1773. doi: 10.1517/13543784.16.11.1753. [DOI] [PubMed] [Google Scholar]
- Downward J. Targeting RAS signalling pathways in cancer therapy. Nat.Rev.Cancer. 2003;3:11–22. doi: 10.1038/nrc969. [DOI] [PubMed] [Google Scholar]
- Dudley DT, Pang L, Decker SJ, Bridges AJ, Saltiel AR. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc.Natl.Acad.Sci.U.S.A. 1995;92:7686–7689. doi: 10.1073/pnas.92.17.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favata MF, Horiuchi KY, Manos EJ, Daulerio AJ, Stradley DA, Feeser WS, Van Dyk DE, Pitts WJ, Earl RA, Hobbs F, Copeland RA, Magolda RL, Scherle PA, Trzaskos JM. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J.Biol.Chem. 1998;273:18623–18632. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- Ficarra R, Tommasini S, Raneri D, Calabro ML, Di Bella MR, Rustichelli C, Gamberini MC, Ficarra P. Study of flavonoids/beta-cyclodextrins inclusion complexes by NMR, FT-IR, DSC, X-ray investigation. J.Pharm.Biomed.Anal. 2002;29:1005–1014. doi: 10.1016/s0731-7085(02)00141-3. [DOI] [PubMed] [Google Scholar]
- Fisher GH, Wellen SL, Klimstra D, Lenczowski JM, Tichelaar JW, Lizak MJ, Whitsett JA, Koretsky A, Varmus HE. Induction and apoptotic regression of lung adenocarcinomas by regulation of a K-Ras transgene in the presence and absence of tumor suppressor genes. Genes Dev. 2001;15:3249–3262. doi: 10.1101/gad.947701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero S, Casanova I, Farre L, Mazo A, Capella G, Mangues R. K-ras codon 12 mutation induces higher level of resistance to apoptosis and predisposition to anchorage-independent growth than codon 13 mutation or proto-oncogene overexpression. Cancer Res. 2000;60:6750–6756. [PubMed] [Google Scholar]
- Guo W, Wu S, Liu J, Fang B. Identification of a small molecule with synthetic lethality for K-ras and protein kinase C iota. Cancer Res. 2008;68:7403–7408. doi: 10.1158/0008-5472.CAN-08-1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Wu S, Wang L, Wang RY, Wei X, Liu J, Fang B. Interruption of RNA processing machinery by a small compound, 1-[(4-chlorophenyl)methyl]-1H-indole-3-carboxaldehyde (oncrasin-1). Mol.Cancer Ther. 2009;8:441–448. doi: 10.1158/1535-7163.MCT-08-0839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L, Mercer K, Greenbaum D, Bronson RT, Crowley D, Tuveson DA, Jacks T. Somatic activation of the K-ras oncogene causes early onset lung cancer in mice. Nature. 2001;410:1111–1116. doi: 10.1038/35074129. [DOI] [PubMed] [Google Scholar]
- Kamata H, Honda S, Maeda S, Chang L, Hirata H, Karin M. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120:649–661. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- Kim SK, Abdelmegeed MA, Novak RF. The mitogen-activated protein kinase kinase (mek) inhibitor PD98059 elevates primary cultured rat hepatocyte glutathione levels independent of inhibiting mek. Drug Metab Dispos. 2006;34:683–689. doi: 10.1124/dmd.105.007666. [DOI] [PubMed] [Google Scholar]
- Knebel A, Rahmsdorf HJ, Ullrich A, Herrlich P. Dephosphorylation of receptor tyrosine kinases as target of regulation by radiation, oxidants or alkylating agents. EMBO J. 1996;15:5314–5325. [PMC free article] [PubMed] [Google Scholar]
- Leevers SJ, Paterson HF, Marshall CJ. Requirement for Ras in Raf activation is overcome by targeting Raf to the plasma membrane. Nature. 1994;369:411–414. doi: 10.1038/369411a0. [DOI] [PubMed] [Google Scholar]
- Liu J, Lin A. Role of JNK activation in apoptosis: a double-edged sword. Cell Res. 2005;15:36–42. doi: 10.1038/sj.cr.7290262. [DOI] [PubMed] [Google Scholar]
- Marais R, Light Y, Paterson HF, Marshall CJ. Ras recruits Raf-1 to the plasma membrane for activation by tyrosine phosphorylation. EMBO J. 1995;14:3136–3145. doi: 10.1002/j.1460-2075.1995.tb07316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merighi S, Benini A, Mirandola P, Gessi S, Varani K, Leung E, Maclennan S, Baraldi PG, Borea PA. Modulation of the Akt/Ras/Raf/MEK/ERK pathway by A(3) adenosine receptor. Purinergic.Signal. 2006;2:627–632. doi: 10.1007/s11302-006-9020-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemunaitis J, Cox J, Meyer W, Courtney A, Mues G. Irinotecan hydrochloride (CPT-11) resistance identified by K-ras mutation in patients with progressive colon cancer after treatment with 5-fluorouracil (5-FU). Am.J.Clin.Oncol. 1997;20:527–529. doi: 10.1097/00000421-199710000-00020. [DOI] [PubMed] [Google Scholar]
- Pacold ME, Suire S, Perisic O, Lara-Gonzalez S, Davis CT, Walker EH, Hawkins PT, Stephens L, Eccleston JF, Williams RL. Crystal structure and functional analysis of Ras binding to its effector phosphoinositide 3-kinase gamma. Cell. 2000;103:931–943. doi: 10.1016/s0092-8674(00)00196-3. [DOI] [PubMed] [Google Scholar]
- Panka DJ, Wang W, Atkins MB, Mier JW. The Raf inhibitor BAY 43-9006 (Sorafenib) induces caspase-independent apoptosis in melanoma cells. Cancer Res. 2006;66:1611–1619. doi: 10.1158/0008-5472.CAN-05-0808. [DOI] [PubMed] [Google Scholar]
- Pauwels B, Korst AE, de Pooter CM, Pattyn GG, Lambrechts HA, Baay MF, Lardon F, Vermorken JB. Comparison of the sulforhodamine B assay and the clonogenic assay for in vitro chemoradiation studies. Cancer Chemother.Pharmacol. 2003;51:221–226. doi: 10.1007/s00280-002-0557-9. [DOI] [PubMed] [Google Scholar]
- Salh BS, Martens J, Hundal RS, Yoganathan N, Charest D, Mui A, Gomez-Munoz A. PD98059 attenuates hydrogen peroxide-induced cell death through inhibition of Jun N-Terminal Kinase in HT29 cells. Mol.Cell Biol.Res.Commun. 2000;4:158–165. doi: 10.1006/mcbr.2001.0271. [DOI] [PubMed] [Google Scholar]
- Sato T, Koike L, Miyata Y, Hirata M, Mimaki Y, Sashida Y, Yano M, Ito A. Inhibition of activator protein-1 binding activity and phosphatidylinositol 3-kinase pathway by nobiletin, a polymethoxy flavonoid, results in augmentation of tissue inhibitor of metalloproteinases-1 production and suppression of production of matrix metalloproteinases-1 and -9 in human fibrosarcoma HT-1080 cells. Cancer Res. 2002;62:1025–1029. [PubMed] [Google Scholar]
- Schubbert S, Bollag G, Lyubynska N, Nguyen H, Kratz CP, Zenker M, Niemeyer CM, Molven A, Shannon K. Biochemical and functional characterization of germ line KRAS mutations. Mol.Cell Biol. 2007;27:7765–7770. doi: 10.1128/MCB.00965-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoba LN, Newman M, Liu W, Lowe WL., Jr. LY 294002, an inhibitor of phosphatidylinositol 3-kinase, inhibits GH-mediated expression of the IGF-I gene in rat hepatocytes. Endocrinology. 2001;142:3980–3986. doi: 10.1210/endo.142.9.8394. [DOI] [PubMed] [Google Scholar]
- Solit DB, Garraway LA, Pratilas CA, Sawai A, Getz G, Basso A, Ye Q, Lobo JM, She Y, Osman I, Golub TR, Sebolt-Leopold J, Sellers WR, Rosen N. BRAF mutation predicts sensitivity to MEK inhibition. Nature. 2006;439:358–362. doi: 10.1038/nature04304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang F, Tang G, Xiang J, Dai Q, Rosner MR, Lin A. The absence of NF-kappaB-mediated inhibition of c-Jun N-terminal kinase activation contributes to tumor necrosis factor alpha-induced apoptosis. Mol.Cell Biol. 2002;22:8571–8579. doi: 10.1128/MCB.22.24.8571-8579.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimmaiah KN, Easton JB, Germain GS, Morton CL, Kamath S, Buolamwini JK, Houghton PJ. Identification of N10-substituted phenoxazines as potent and specific inhibitors of Akt signaling. J.Biol.Chem. 2005;280:31924–31935. doi: 10.1074/jbc.M507057200. [DOI] [PubMed] [Google Scholar]
- Tournier C, Hess P, Yang DD, Xu J, Turner TK, Nimnual A, Bar-Sagi D, Jones SN, Flavell RA, Davis RJ. Requirement of JNK for stress-induced activation of the cytochrome c-mediated death pathway. Science. 2000;288:870–874. doi: 10.1126/science.288.5467.870. [DOI] [PubMed] [Google Scholar]
- Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat.Rev.Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- Wei H, Cai Q, Rahn RO. Inhibition of UV light- and Fenton reaction-induced oxidative DNA damage by the soybean isoflavone genistein. Carcinogenesis. 1996;17:73–77. doi: 10.1093/carcin/17.1.73. [DOI] [PubMed] [Google Scholar]
- Wei X, Guo W, Wu S, Wang L, Lu Y, Xu B, Liu J, Fang B. Inhibiting JNK dephosphorylation and induction of apoptosis by novel anticancer agent NSC-741909 in cancer cells. J.Biol.Chem. 2009;284:16948–16955. doi: 10.1074/jbc.M109.010256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston CR, Davis RJ. The JNK signal transduction pathway. Curr.Opin.Genet.Dev. 2002;12:14–21. doi: 10.1016/s0959-437x(01)00258-1. [DOI] [PubMed] [Google Scholar]
- Zheng C, Xiang J, Hunter T, Lin A. The JNKK2-JNK1 fusion protein acts as a constitutively active c-Jun kinase that stimulates c-Jun transcription activity. J.Biol.Chem. 1999;274:28966–28971. doi: 10.1074/jbc.274.41.28966. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.