Figure 2. Solid agar growth of the alkali-metal-cation sensitive BW31a yeast strain expressing human NHAoc/NHA2.
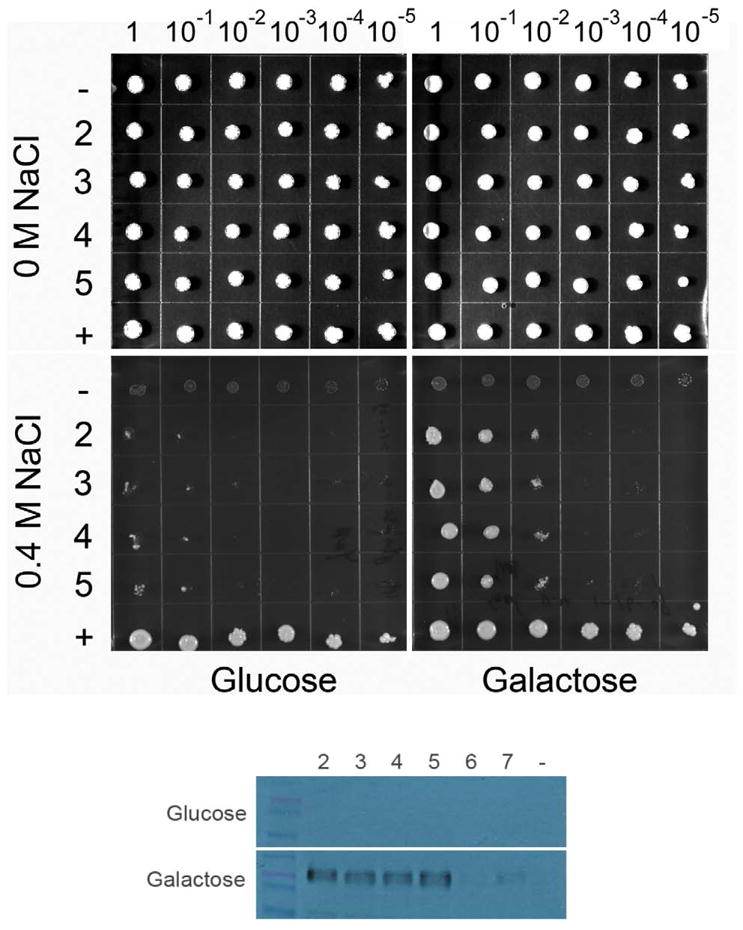
(top) Ten-fold serial dilutions of yeast suspensions (1 – 10−5) were spotted on YNB plates supplemented with NaCl and 2% glucose or 2% galactose, as indicated. Cells transformed with the four independent plasmids that were high expressers of NHAoc/NHA2 from the GAL1 promoters were tested (rows 2, 3, 4 and 5). Cells transformed with empty vector (row -) and cells expressing their own NHA1 antiporter (pNHA1–985GFP, row +) were used as negative and positive controls, respectively. (bottom) Western Blot analysis of BW31a cells transformed with NHAoc/NHA2 expression plasmids (lanes 2 – 7). Blots were incubated with a V5 antibody. Clones 2–5 were high expressers of NHAoc/NHA2 and were therefore used in the subsequent experiment seen in the top panel of this figure. Cells transformed with an empty vector (lane -) were used as negative control. A ~60 KDa protein, corresponding to NHAoc/NHA2 – V5 fusion protein, can be detected in lanes 2–5.
