Abstract
The title compound, C26H22O4, is a derivative of 1,4-bis(phenylethynyl)benzene substituted by four methoxy groups on the terminal benzene rings. The molecule is almost planar with an r.m.s. deviation of 0.266 Å. The dihedral angles between the two terminal benzene rings and the central benzene ring are 7.96 (6) and 13.32 (7)°. In the crystal structure, molecules aggregate via C—H⋯O interactions, forming molecular tapes along the a axis, which aggregate to form a herring-bone structure.
Related literature
For the crystal structure of 1,4-bis[(2,6-dimethoxyphenyl)ethynyl]benzene, see: Ono et al. (2008 ▶). For related sructures, including a 1,4-bis(phenylethynyl)benzene system, see: Watt et al. (2004 ▶); Li et al. (1998 ▶); Filatov & Petrukhina (2005 ▶).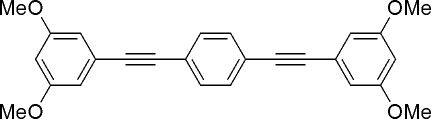
Experimental
Crystal data
C26H22O4
M r = 398.44
Monoclinic,

a = 8.8980 (5) Å
b = 19.4610 (8) Å
c = 12.2820 (5) Å
β = 100.607 (1)°
V = 2090.46 (17) Å3
Z = 4
Mo Kα radiation
μ = 0.09 mm−1
T = 173 (1) K
0.30 × 0.25 × 0.15 mm
Data collection
Rigaku Mercury CCD diffractometer
Absorption correction: none
15238 measured reflections
4638 independent reflections
3914 reflections with I > 2σ(I)
R int = 0.024
Refinement
R[F 2 > 2σ(F 2)] = 0.048
wR(F 2) = 0.140
S = 1.11
4638 reflections
271 parameters
H-atom parameters constrained
Δρmax = 0.26 e Å−3
Δρmin = −0.17 e Å−3
Data collection: CrystalClear (Rigaku, 2001 ▶); cell refinement: CrystalClear; data reduction: CrystalClear; program(s) used to solve structure: SIR2002 (Burla et al., 2003 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: PLATON (Spek, 2003 ▶) and Mercury (Macrae et al., 2006 ▶); software used to prepare material for publication: WinGX (Farrugia, 1999 ▶).
Supplementary Material
Crystal structure: contains datablocks I, global. DOI: 10.1107/S160053680900155X/ci2753sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S160053680900155X/ci2753Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C11—H11⋯O4i | 0.95 | 2.42 | 3.3511 (17) | 167 |
| C14—H14⋯O2ii | 0.95 | 2.37 | 3.2758 (16) | 160 |
Symmetry codes: (i)  ; (ii)
; (ii)  .
.
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research (grant No. 19550034) from the Ministry of Education, Culture, Sports, Science and Technology, Japan. The authors thank the Instrument Center of the Institute for Molecular Science for the X-ray structure analysis.
supplementary crystallographic information
Comment
The synthetic research of ethynylated aromatic compounds has attracted considerable attention because of interests in their molecular structures, optical properties, and molecular electronics. Among these ethynylated aromatic compounds, 1,4-bis(phenylethynyl)benzene derivatives have been extensively studied. These compounds have stiff, linear molecular structures and are used as building blocks in the applications. Recently, we found that 1,4-bis[(2,6-dimethoxyphenyl)ethynyl]benzene, (II), formed a zigzag molecular network in the crystal (Ono et al., 2008). The crystal structure is different from those of 1,4-bis(phenylethynyl)benzene derivatives (Watt et al., 2004; Li et al., 1998; Filatov & Petrukhina, 2005). With regard to this, we investigated the molecular and crystal structure of the title compound, (I), which is a regioisomer of (II). The substitution effect of four methoxy groups at the terminal benzene rings was studied.
The molecular structure of (I) is shown in Fig. 1. The molecule is almost planar with an r.m.s deviation of 0.266 Å. The dihedral angles between the terminal benzene rings and the central benzene ring are 7.96 (6)° (C1–C6) and 13.32 (7)° (C17–C22). The methoxy groups are coplanar with the attached benzene rings.
The crystal structure is characterized by a molecular tape along the a axis formed by C—H···O interactions (Table 1 and Fig. 2). The molecular tapes aggregate to form a herring-bone-type structure, as shown in Fig.3. The crystal structure of (I) is different from that of (II). The crystal structures of (I) and (II) indicate that the methoxy groups at terminal benzene rings play an important role in the crystal packing.
Experimental
The title compound (I) was prepared as follows: Tetrakis(triphenylphosphine)palladium(0) [Pd(PPh3)4] (52 mg, 0.045 mmol) was added to a mixture of 1-ethynyl-3,5-dimethoxybenzene (0.39 g, 2.4 mmol), 1,4-diiodobenzene (0.39 g, 1.2 mmol) and copper(I) iodide (5 mg, 0.03 mmol) in dry triethylamine (7 ml) under nitrogen. The reaction mixture was stirred for 18 h at 353 K. After removal of the solvent, dichloromethane (20 ml) and aqueous disodium ethylenediaminetetraacetate (Na2edta) solution (5%, 20 ml) were added. The organic layer was separated and washed with water (20 ml). The organic solution was dried over Na2SO4 and concentrated. The residue was chromatographed on silica gel (CH2Cl2) to afford the title compound (0.23 g, 49%) as a yellow powder. Yellow crystals of the compound, suitable for X-ray analysis were grown from an ethanol solution.
Refinement
All H atoms were placed in geometrically calculated positions, with C-H = 0.95 (aromatic) and 0.98 Å (methyl) and Uiso(H) = 1.2Ueq(C) (aromatic) and 1.5Ueq(C) (methyl), and refined using a riding model.
Figures
Fig. 1.
The molecular structure of (I), with atom labels and 50% probability displacement ellipsoids for non-H atoms and H atoms are shown as small spheres of arbitrary radii.
Fig. 2.
Partial packing diagram of (I), showing a molecular tape along the a axis.
Fig. 3.
The packing diagram of (I), showing herringbone-type network on the bc plane.
Crystal data
| C26H22O4 | F(000) = 840 |
| Mr = 398.44 | Dx = 1.266 Mg m−3 |
| Monoclinic, P21/a | Melting point: 431 K |
| Hall symbol: -P 2yab | Mo Kα radiation, λ = 0.71073 Å |
| a = 8.8980 (5) Å | Cell parameters from 5437 reflections |
| b = 19.4610 (8) Å | θ = 3.1–27.5° |
| c = 12.2820 (5) Å | µ = 0.09 mm−1 |
| β = 100.607 (1)° | T = 173 K |
| V = 2090.46 (17) Å3 | Block, yellow |
| Z = 4 | 0.30 × 0.25 × 0.15 mm |
Data collection
| Rigaku Mercury CCD diffractometer | 3914 reflections with I > 2σ(I) |
| Radiation source: Rotating Anode | Rint = 0.024 |
| Graphite Monochromator | θmax = 27.5°, θmin = 3.1° |
| Detector resolution: 14.7059 pixels mm-1 | h = −11→10 |
| φ and ω scans | k = −22→25 |
| 15238 measured reflections | l = −15→12 |
| 4638 independent reflections |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.048 | H-atom parameters constrained |
| wR(F2) = 0.140 | w = 1/[σ2(Fo2) + (0.0818P)2 + 0.1522P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.11 | (Δ/σ)max = 0.001 |
| 4638 reflections | Δρmax = 0.26 e Å−3 |
| 271 parameters | Δρmin = −0.17 e Å−3 |
| 0 restraints |
Special details
| Experimental. IR (KBr, cm-1): 1605, 1580, 1345, 1254, 1202, 1161, 1065, 841; 1H NMR (CDCl3, δ p.p.m.): 3.81 (s, 12H), 6.48 (t, J = 2.3 Hz, 2H), 6.70 (d, J = 2.3 Hz, 4H), 7.51 (s, 4H); 13C NMR (CDCl3, δ p.p.m.): 55.3, 88.6, 91.3, 102.0, 109.4, 123.0, 124.3, 131.6, 160.6; MS (EI): m/z 398 (M+), 199. |
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| C1 | 0.04347 (14) | 0.17522 (6) | 0.71287 (10) | 0.0266 (3) | |
| C2 | −0.11459 (14) | 0.17481 (6) | 0.67760 (11) | 0.0266 (3) | |
| H2 | −0.1799 | 0.1980 | 0.7185 | 0.032* | |
| C3 | −0.17430 (13) | 0.13932 (6) | 0.58033 (11) | 0.0265 (3) | |
| C4 | −0.07979 (14) | 0.10623 (6) | 0.51948 (11) | 0.0277 (3) | |
| H4 | −0.1225 | 0.0824 | 0.4535 | 0.033* | |
| C5 | 0.07839 (14) | 0.10787 (6) | 0.55512 (10) | 0.0261 (3) | |
| C6 | 0.14059 (14) | 0.14194 (6) | 0.65309 (11) | 0.0283 (3) | |
| H6 | 0.2480 | 0.1424 | 0.6787 | 0.034* | |
| C7 | 0.17340 (14) | 0.07642 (6) | 0.48619 (11) | 0.0288 (3) | |
| C8 | 0.24292 (14) | 0.05168 (7) | 0.42138 (11) | 0.0288 (3) | |
| C9 | 0.32492 (14) | 0.02180 (6) | 0.34289 (10) | 0.0262 (3) | |
| C10 | 0.24597 (14) | −0.01164 (7) | 0.24864 (11) | 0.0286 (3) | |
| H10 | 0.1377 | −0.0150 | 0.2371 | 0.034* | |
| C11 | 0.32439 (14) | −0.03986 (7) | 0.17214 (11) | 0.0303 (3) | |
| H11 | 0.2695 | −0.0621 | 0.1081 | 0.036* | |
| C12 | 0.48372 (14) | −0.03594 (6) | 0.18839 (10) | 0.0273 (3) | |
| C13 | 0.56275 (14) | −0.00329 (6) | 0.28348 (11) | 0.0297 (3) | |
| H13 | 0.6712 | −0.0009 | 0.2959 | 0.036* | |
| C14 | 0.48439 (14) | 0.02550 (7) | 0.35943 (11) | 0.0295 (3) | |
| H14 | 0.5392 | 0.0479 | 0.4233 | 0.035* | |
| C15 | 0.56748 (14) | −0.06453 (7) | 0.10994 (11) | 0.0305 (3) | |
| C16 | 0.64138 (15) | −0.08764 (7) | 0.04662 (11) | 0.0313 (3) | |
| C17 | 0.73414 (14) | −0.11338 (6) | −0.02860 (11) | 0.0289 (3) | |
| C18 | 0.67256 (14) | −0.15780 (7) | −0.11433 (11) | 0.0309 (3) | |
| H18 | 0.5689 | −0.1720 | −0.1231 | 0.037* | |
| C19 | 0.76419 (14) | −0.18118 (7) | −0.18695 (11) | 0.0289 (3) | |
| C20 | 0.91672 (14) | −0.16179 (7) | −0.17484 (11) | 0.0289 (3) | |
| H20 | 0.9792 | −0.1787 | −0.2238 | 0.035* | |
| C21 | 0.97568 (14) | −0.11696 (7) | −0.08931 (11) | 0.0310 (3) | |
| C22 | 0.88622 (14) | −0.09254 (7) | −0.01627 (11) | 0.0316 (3) | |
| H22 | 0.9283 | −0.0619 | 0.0416 | 0.038* | |
| C23 | 0.02302 (18) | 0.24526 (8) | 0.87014 (12) | 0.0434 (4) | |
| H23A | 0.0889 | 0.2677 | 0.9328 | 0.065* | |
| H23B | −0.0440 | 0.2122 | 0.8980 | 0.065* | |
| H23C | −0.0392 | 0.2800 | 0.8247 | 0.065* | |
| C24 | −0.43253 (15) | 0.17035 (8) | 0.58918 (13) | 0.0409 (4) | |
| H24A | −0.5365 | 0.1618 | 0.5488 | 0.061* | |
| H24B | −0.4108 | 0.2197 | 0.5890 | 0.061* | |
| H24C | −0.4236 | 0.1543 | 0.6657 | 0.061* | |
| C25 | 0.77935 (17) | −0.24753 (8) | −0.34851 (12) | 0.0398 (3) | |
| H25A | 0.7151 | −0.2772 | −0.4025 | 0.060* | |
| H25B | 0.8679 | −0.2737 | −0.3107 | 0.060* | |
| H25C | 0.8146 | −0.2082 | −0.3868 | 0.060* | |
| C26 | 1.22202 (17) | −0.11600 (9) | −0.14105 (15) | 0.0506 (4) | |
| H26A | 1.3229 | −0.0950 | −0.1178 | 0.076* | |
| H26B | 1.1803 | −0.1025 | −0.2175 | 0.076* | |
| H26C | 1.2316 | −0.1661 | −0.1366 | 0.076* | |
| O1 | 0.11541 (10) | 0.21008 (5) | 0.80454 (8) | 0.0360 (2) | |
| O2 | −0.32622 (10) | 0.13442 (5) | 0.53688 (8) | 0.0352 (2) | |
| O3 | 0.69301 (10) | −0.22363 (5) | −0.26936 (9) | 0.0396 (3) | |
| O4 | 1.12292 (11) | −0.09341 (6) | −0.07075 (9) | 0.0477 (3) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| C1 | 0.0340 (6) | 0.0247 (6) | 0.0213 (6) | 0.0006 (5) | 0.0059 (5) | −0.0006 (5) |
| C2 | 0.0318 (6) | 0.0241 (6) | 0.0263 (7) | 0.0011 (5) | 0.0113 (5) | −0.0004 (5) |
| C3 | 0.0273 (6) | 0.0244 (6) | 0.0287 (7) | −0.0013 (4) | 0.0073 (5) | 0.0015 (5) |
| C4 | 0.0345 (6) | 0.0257 (6) | 0.0235 (6) | −0.0007 (5) | 0.0068 (5) | −0.0036 (5) |
| C5 | 0.0322 (6) | 0.0232 (6) | 0.0249 (6) | 0.0034 (5) | 0.0106 (5) | 0.0016 (5) |
| C6 | 0.0280 (6) | 0.0281 (6) | 0.0291 (7) | 0.0025 (5) | 0.0058 (5) | −0.0005 (5) |
| C7 | 0.0314 (6) | 0.0279 (6) | 0.0277 (7) | 0.0016 (5) | 0.0071 (5) | −0.0007 (5) |
| C8 | 0.0324 (6) | 0.0280 (6) | 0.0266 (7) | 0.0020 (5) | 0.0069 (5) | −0.0004 (5) |
| C9 | 0.0309 (6) | 0.0248 (6) | 0.0243 (6) | 0.0033 (5) | 0.0089 (5) | 0.0012 (5) |
| C10 | 0.0266 (6) | 0.0331 (7) | 0.0265 (7) | 0.0027 (5) | 0.0061 (5) | −0.0005 (5) |
| C11 | 0.0340 (6) | 0.0338 (7) | 0.0226 (6) | 0.0014 (5) | 0.0042 (5) | −0.0045 (5) |
| C12 | 0.0330 (6) | 0.0269 (6) | 0.0238 (6) | 0.0043 (5) | 0.0103 (5) | 0.0000 (5) |
| C13 | 0.0277 (6) | 0.0333 (7) | 0.0293 (7) | 0.0007 (5) | 0.0083 (5) | −0.0012 (5) |
| C14 | 0.0325 (6) | 0.0316 (6) | 0.0248 (7) | −0.0015 (5) | 0.0064 (5) | −0.0051 (5) |
| C15 | 0.0346 (6) | 0.0303 (7) | 0.0278 (7) | 0.0033 (5) | 0.0086 (5) | −0.0006 (5) |
| C16 | 0.0358 (6) | 0.0316 (7) | 0.0278 (7) | 0.0036 (5) | 0.0092 (5) | −0.0007 (5) |
| C17 | 0.0344 (6) | 0.0299 (6) | 0.0238 (6) | 0.0062 (5) | 0.0089 (5) | 0.0002 (5) |
| C18 | 0.0286 (6) | 0.0338 (7) | 0.0322 (7) | −0.0007 (5) | 0.0109 (5) | −0.0034 (6) |
| C19 | 0.0317 (6) | 0.0294 (6) | 0.0259 (7) | −0.0011 (5) | 0.0065 (5) | −0.0044 (5) |
| C20 | 0.0296 (6) | 0.0330 (7) | 0.0255 (7) | 0.0018 (5) | 0.0091 (5) | −0.0038 (5) |
| C21 | 0.0286 (6) | 0.0363 (7) | 0.0285 (7) | −0.0008 (5) | 0.0057 (5) | −0.0029 (5) |
| C22 | 0.0356 (7) | 0.0348 (7) | 0.0244 (7) | 0.0003 (5) | 0.0057 (5) | −0.0071 (5) |
| C23 | 0.0512 (8) | 0.0486 (9) | 0.0291 (8) | 0.0112 (7) | 0.0037 (6) | −0.0135 (7) |
| C24 | 0.0286 (6) | 0.0471 (9) | 0.0487 (9) | 0.0001 (6) | 0.0112 (6) | −0.0110 (7) |
| C25 | 0.0459 (8) | 0.0429 (8) | 0.0324 (8) | −0.0052 (6) | 0.0121 (6) | −0.0150 (6) |
| C26 | 0.0339 (7) | 0.0718 (12) | 0.0499 (10) | −0.0131 (7) | 0.0179 (7) | −0.0190 (8) |
| O1 | 0.0364 (5) | 0.0429 (6) | 0.0274 (5) | 0.0056 (4) | 0.0023 (4) | −0.0123 (4) |
| O2 | 0.0271 (4) | 0.0380 (5) | 0.0403 (6) | −0.0022 (4) | 0.0062 (4) | −0.0102 (4) |
| O3 | 0.0361 (5) | 0.0459 (6) | 0.0390 (6) | −0.0091 (4) | 0.0123 (4) | −0.0198 (5) |
| O4 | 0.0302 (5) | 0.0698 (7) | 0.0446 (7) | −0.0122 (5) | 0.0112 (4) | −0.0260 (6) |
Geometric parameters (Å, °)
| C1—O1 | 1.3684 (15) | C17—C22 | 1.3940 (18) |
| C1—C6 | 1.3924 (16) | C17—C18 | 1.3942 (18) |
| C1—C2 | 1.3933 (17) | C18—C19 | 1.3911 (16) |
| C2—C3 | 1.3975 (18) | C18—H18 | 0.95 |
| C2—H2 | 0.95 | C19—O3 | 1.3683 (15) |
| C3—O2 | 1.3622 (15) | C19—C20 | 1.3900 (17) |
| C3—C4 | 1.3826 (16) | C20—C21 | 1.3917 (18) |
| C4—C5 | 1.3953 (17) | C20—H20 | 0.95 |
| C4—H4 | 0.95 | C21—O4 | 1.3670 (15) |
| C5—C6 | 1.3960 (18) | C21—C22 | 1.3882 (17) |
| C5—C7 | 1.4386 (16) | C22—H22 | 0.95 |
| C6—H6 | 0.95 | C23—O1 | 1.4274 (16) |
| C7—C8 | 1.1956 (17) | C23—H23A | 0.98 |
| C8—C9 | 1.4348 (16) | C23—H23B | 0.98 |
| C9—C14 | 1.3977 (17) | C23—H23C | 0.98 |
| C9—C10 | 1.3991 (18) | C24—O2 | 1.4213 (15) |
| C10—C11 | 1.3831 (17) | C24—H24A | 0.98 |
| C10—H10 | 0.95 | C24—H24B | 0.98 |
| C11—C12 | 1.3969 (17) | C24—H24C | 0.98 |
| C11—H11 | 0.95 | C25—O3 | 1.4236 (16) |
| C12—C13 | 1.3999 (18) | C25—H25A | 0.98 |
| C12—C15 | 1.4345 (16) | C25—H25B | 0.98 |
| C13—C14 | 1.3820 (17) | C25—H25C | 0.98 |
| C13—H13 | 0.95 | C26—O4 | 1.4130 (17) |
| C14—H14 | 0.95 | C26—H26A | 0.98 |
| C15—C16 | 1.1949 (18) | C26—H26B | 0.98 |
| C16—C17 | 1.4375 (17) | C26—H26C | 0.98 |
| O1—C1—C6 | 115.01 (10) | C19—C18—C17 | 119.46 (11) |
| O1—C1—C2 | 123.44 (11) | C19—C18—H18 | 120.3 |
| C6—C1—C2 | 121.49 (11) | C17—C18—H18 | 120.3 |
| C1—C2—C3 | 118.14 (11) | O3—C19—C20 | 123.57 (11) |
| C1—C2—H2 | 120.9 | O3—C19—C18 | 115.19 (11) |
| C3—C2—H2 | 120.9 | C20—C19—C18 | 121.24 (12) |
| O2—C3—C4 | 114.46 (11) | C19—C20—C21 | 118.42 (11) |
| O2—C3—C2 | 124.28 (11) | C19—C20—H20 | 120.8 |
| C4—C3—C2 | 121.26 (11) | C21—C20—H20 | 120.8 |
| C3—C4—C5 | 119.91 (11) | O4—C21—C22 | 115.05 (12) |
| C3—C4—H4 | 120.0 | O4—C21—C20 | 123.55 (11) |
| C5—C4—H4 | 120.0 | C22—C21—C20 | 121.40 (11) |
| C4—C5—C6 | 119.88 (11) | C21—C22—C17 | 119.40 (12) |
| C4—C5—C7 | 118.29 (11) | C21—C22—H22 | 120.3 |
| C6—C5—C7 | 121.78 (11) | C17—C22—H22 | 120.3 |
| C1—C6—C5 | 119.30 (11) | O1—C23—H23A | 109.5 |
| C1—C6—H6 | 120.4 | O1—C23—H23B | 109.5 |
| C5—C6—H6 | 120.4 | H23A—C23—H23B | 109.5 |
| C8—C7—C5 | 174.44 (14) | O1—C23—H23C | 109.5 |
| C7—C8—C9 | 179.42 (15) | H23A—C23—H23C | 109.5 |
| C14—C9—C10 | 119.07 (11) | H23B—C23—H23C | 109.5 |
| C14—C9—C8 | 120.64 (11) | O2—C24—H24A | 109.5 |
| C10—C9—C8 | 120.29 (11) | O2—C24—H24B | 109.5 |
| C11—C10—C9 | 120.50 (11) | H24A—C24—H24B | 109.5 |
| C11—C10—H10 | 119.8 | O2—C24—H24C | 109.5 |
| C9—C10—H10 | 119.8 | H24A—C24—H24C | 109.5 |
| C10—C11—C12 | 120.45 (11) | H24B—C24—H24C | 109.5 |
| C10—C11—H11 | 119.8 | O3—C25—H25A | 109.5 |
| C12—C11—H11 | 119.8 | O3—C25—H25B | 109.5 |
| C11—C12—C13 | 119.00 (11) | H25A—C25—H25B | 109.5 |
| C11—C12—C15 | 121.46 (11) | O3—C25—H25C | 109.5 |
| C13—C12—C15 | 119.54 (11) | H25A—C25—H25C | 109.5 |
| C14—C13—C12 | 120.58 (11) | H25B—C25—H25C | 109.5 |
| C14—C13—H13 | 119.7 | O4—C26—H26A | 109.5 |
| C12—C13—H13 | 119.7 | O4—C26—H26B | 109.5 |
| C13—C14—C9 | 120.39 (12) | H26A—C26—H26B | 109.5 |
| C13—C14—H14 | 119.8 | O4—C26—H26C | 109.5 |
| C9—C14—H14 | 119.8 | H26A—C26—H26C | 109.5 |
| C16—C15—C12 | 177.95 (14) | H26B—C26—H26C | 109.5 |
| C15—C16—C17 | 177.90 (14) | C1—O1—C23 | 118.13 (10) |
| C22—C17—C18 | 120.08 (11) | C3—O2—C24 | 118.91 (10) |
| C22—C17—C16 | 119.31 (12) | C19—O3—C25 | 117.90 (10) |
| C18—C17—C16 | 120.60 (11) | C21—O4—C26 | 118.72 (11) |
| O1—C1—C2—C3 | 177.69 (11) | C8—C9—C14—C13 | −179.92 (12) |
| C6—C1—C2—C3 | 0.59 (18) | C22—C17—C18—C19 | 0.2 (2) |
| C1—C2—C3—O2 | 179.60 (11) | C16—C17—C18—C19 | 179.07 (12) |
| C1—C2—C3—C4 | −0.90 (18) | C17—C18—C19—O3 | −178.99 (12) |
| O2—C3—C4—C5 | 179.63 (11) | C17—C18—C19—C20 | 0.8 (2) |
| C2—C3—C4—C5 | 0.08 (18) | O3—C19—C20—C21 | 178.44 (12) |
| C3—C4—C5—C6 | 1.06 (18) | C18—C19—C20—C21 | −1.4 (2) |
| C3—C4—C5—C7 | −176.30 (11) | C19—C20—C21—O4 | −178.72 (13) |
| O1—C1—C6—C5 | −176.80 (11) | C19—C20—C21—C22 | 0.9 (2) |
| C2—C1—C6—C5 | 0.53 (19) | O4—C21—C22—C17 | 179.75 (12) |
| C4—C5—C6—C1 | −1.36 (18) | C20—C21—C22—C17 | 0.1 (2) |
| C7—C5—C6—C1 | 175.90 (11) | C18—C17—C22—C21 | −0.6 (2) |
| C14—C9—C10—C11 | −0.84 (19) | C16—C17—C22—C21 | −179.55 (12) |
| C8—C9—C10—C11 | 179.30 (12) | C6—C1—O1—C23 | −179.78 (12) |
| C9—C10—C11—C12 | 0.59 (19) | C2—C1—O1—C23 | 2.94 (18) |
| C10—C11—C12—C13 | 0.28 (19) | C4—C3—O2—C24 | −175.99 (12) |
| C10—C11—C12—C15 | −179.69 (12) | C2—C3—O2—C24 | 3.55 (18) |
| C11—C12—C13—C14 | −0.90 (19) | C20—C19—O3—C25 | −1.8 (2) |
| C15—C12—C13—C14 | 179.07 (12) | C18—C19—O3—C25 | 178.03 (12) |
| C12—C13—C14—C9 | 0.65 (19) | C22—C21—O4—C26 | 179.71 (14) |
| C10—C9—C14—C13 | 0.22 (19) | C20—C21—O4—C26 | −0.6 (2) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C11—H11···O4i | 0.95 | 2.42 | 3.3511 (17) | 167 |
| C14—H14···O2ii | 0.95 | 2.37 | 3.2758 (16) | 160 |
Symmetry codes: (i) x−1, y, z; (ii) x+1, y, z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: CI2753).
References
- Burla, M. C., Camalli, M., Carrozzini, B., Cascarano, G. L., Giacovazzo, C., Polidori, G. & Spagna, R. (2003). J. Appl. Cryst.36, 1103.
- Farrugia, L. J. (1999). J. Appl. Cryst.32, 837–838.
- Filatov, A. S. & Petrukhina, M. A. (2005). Acta Cryst. C61, o193–o194. [DOI] [PubMed]
- Li, H., Powell, D. R., Firman, T. K. & West, R. (1998). Macromolecules, 31, 1093–1098.
- Macrae, C. F., Edgington, P. R., McCabe, P., Pidcock, E., Shields, G. P., Taylor, R., Towler, M. & van de Streek, J. (2006). J. Appl. Cryst.39, 453–457.
- Ono, K., Tsukamoto, K., Tomura, M. & Saito, K. (2008). Acta Cryst. E64, o1069. [DOI] [PMC free article] [PubMed]
- Rigaku (2001). CrystalClear Rigaku Corporation, Tokyo, Japan.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Spek, A. L. (2003). J. Appl. Cryst.36, 7–13.
- Watt, S. W., Dai, C., Scott, A. J., Burke, J. M., Thomas, R. Ll., Collings, J. C., Viney, C., Clegg, W. & Marder, T. B. (2004). Angew. Chem. Int. Ed.43, 3061–3063. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, global. DOI: 10.1107/S160053680900155X/ci2753sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S160053680900155X/ci2753Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report





