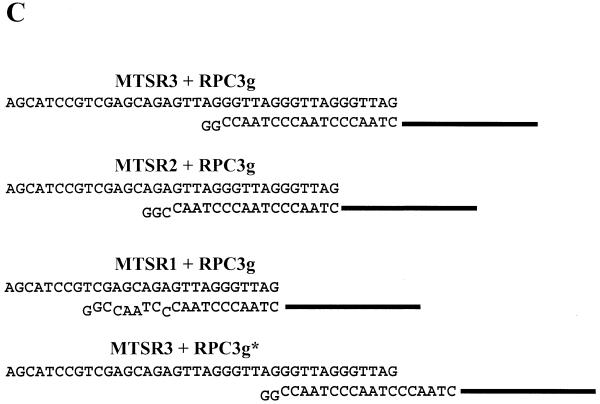
Figure 3.
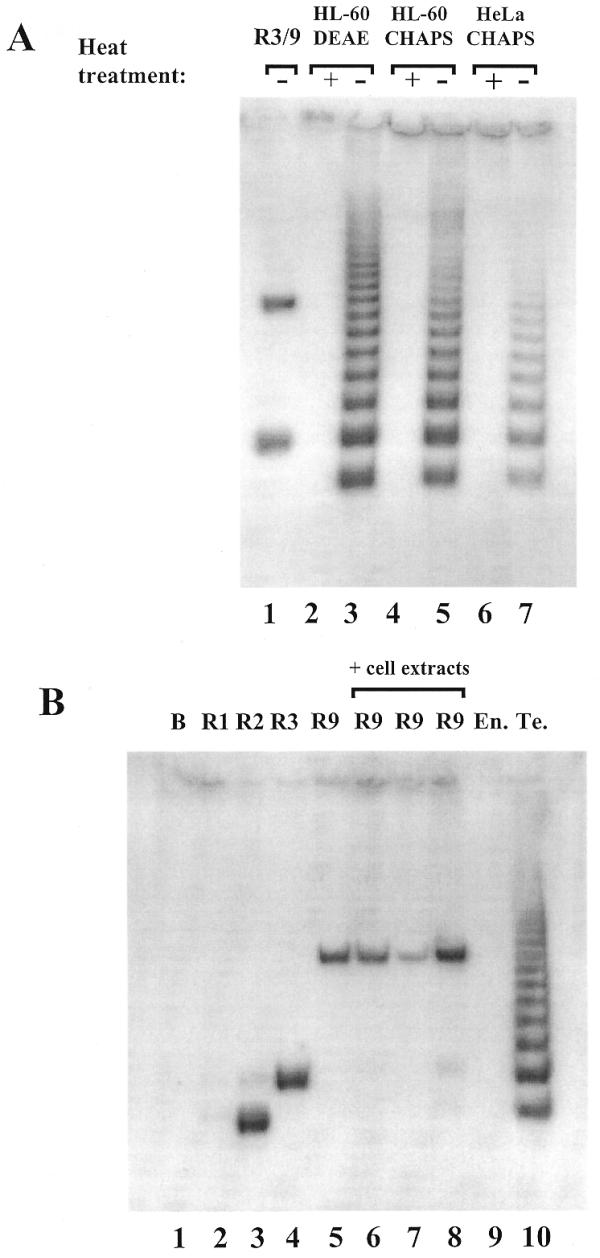
(A) Detection of telomerase activity with the new primer combination, MTS/RPC3g/RP. Partially purified enzyme (HL-60 DEAE) containing 100 ng protein (lanes 2 and 3) and crude extracts from HL-60 (lanes 4 and 5) and HeLa (lanes 6 and 7) cells containing 1000 ng protein were used as telomerase enzymes. Heat-inactivated samples acted as controls (lanes 2, 4 and 6). As a positive control, 0.1 amol MTSR3 and 0.1 amol MTSR9 were amplified (lane 1). (B) Amplification of MTSR1, MTSR2, MTSR3 and MTSR9 in the presence of different cell extracts. Lane 1, no template was added (blank); lanes 2–4, 0.1 amol MTSR1, MTSR2 and MTSR3, respectively, were amplified; lane 5, amplification of 0.1 amol MTSR9. Before PCR amplification 0.1 amol MTSR9 was incubated with 100 ng heat-inactivated telomerase enzyme (HL-60 DEAE) (lane 6), 1000 ng crude cell extract (HL-60 CHAPS) (lane 7) or 2000 ng extracts from primer endothelial cells (lane 8). As control reactions 2000 ng endothel cell extract (lane 9) and 100 ng partially purified telomerase enzyme (HL-60 DEAE) (lane 10) were used without MTSR9. (C) Annealing RPC3g with artificial telomerase products. The structures shown in the first two schemes (MTSR3+RPC3g and MTSR2+RPC3g) can support amplification with the MTS/RPC3g/RP primer set. The structures shown in the third (MTSR1+RPC3g) and fourth (MTSR3+RPC3g*) schemes are not stable under the assay conditions, producing no amplified products.