Abstract
Introduction: The age-specific prevalence of polycystic ovaries (PCO), as defined by the Rotterdam criteria, among normal ovulatory women, has not yet been reported. It is also uncertain whether these women differ from their peers in the hormonal or metabolic profile.
Methods: A total of 262 ovulatory Caucasian women aged 25–45 yr, enrolled in a community-based ovarian aging study (OVA), underwent transvaginal ultrasound assessment of ovarian volume and antral follicle count (AFC) in the early follicular phase and were categorized as to whether they met the Rotterdam definition of PCO by AFC (≥12 in one ovary) and/or by volume (>10 cm3 for one ovary). The effect of age on prevalence of PCO was assessed. Serum hormones and metabolic measures were compared between women meeting each element of the Rotterdam criterion and those without PCO using age-adjusted linear regressions.
Results: The prevalence of PCO by AFC was 32% and decreased with age. Those with PCO by AFC had lower FSH; higher anti-Müllerian hormone, estrone, dehydroepiandrostenedione sulfate, and free androgen index; and slightly higher total testosterone than those without PCO. However, slightly higher body mass index and waist circumference were the only metabolic differences. Women with PCO by volume had higher anti-Müllerian hormone and free androgen index but did not differ in any other hormonal or metabolic parameter.
Discussion: PCO is a common, age-dependent finding among ovulatory women. These women lack the metabolic abnormalities seen in PCO syndrome. Isolated PCO in an ovulatory woman is not an indication for metabolic evaluation.
Rotterdam polycystic ovaries are found in 30% of ovulatory women ages 25–45 and are associated with increased body mass index, anti-Müllerian hormone and androgens, but no metabolic differences.
The significance of ovarian morphology to the diagnosis of polycystic ovary syndrome (PCOS) is a topic of controversy. Although the diagnostic criteria defined at the National Institutes of Health (NIH) in 1990 did not include ovarian morphology, the Rotterdam criteria of 2003 incorporated this feature, requiring at least two of three factors: clinical or biochemical hyperandrogenism, oligo- or anovulation, and polycystic ovaries. The polycystic ovary (PCO) was defined as 12 or more follicles 2–9 mm in either ovary and/or an ovarian volume of 10 cm3 or greater (1).
The primary basis for the antral follicle count (AFC) in the definition was a study including 214 women with PCOS and 112 ovulatory, but infertile, women with normal ovarian volume (2). PCOS was defined as one clinical factor (hirsutism or oligomenorrhea) plus either elevation of one serum value [LH, testosterone (T) and/or androstenedione] or an ovarian area greater than 5.5 cm2. Herein, a threshold of 12 follicles of 2–9 mm per ovary optimized discrimination between those with and without PCOS, with a sensitivity of 75% and a specificity of 99%. It is important to note that ovulatory, nonhirsute women with polycystic ovaries were excluded, and their inclusion would have decreased this specificity. The Rotterdam volume threshold of 10 cm3 was reached by consensus upon review of literature suggesting the upper limit of normal ovarian volume to be between 8 and 10.7 cm3 (1,3,4).
Before 2003, the most commonly applied characterization of PCO was that defined by Adams: 10 or more follicles 2–8 mm in diameter, peripherally arranged around a dense area of stroma (5). This common ultrasound finding has a population prevalence between 14 and 33%, and 7–24% among women with regular menses not using oral contraception (6,7,8,9,10,11). It is well established that AFC decreases with age during the reproductive years in normal women (12,13,14,15,16), and it is suggested the same is true in women with PCOS (17). Older women are less likely than younger women to display PCO by the Adams criteria (11). In a recent study, using the Rotterdam criteria for PCO, a prevalence of 63% among women 18–40 yr, decreasing with age, was noted. However, this study was limited by its inclusion of women with oligo-anovulatory cycles and/or hyperandrogenism (18).
Women with PCOS often have increased serum androgens and are at increased risk for metabolic syndrome (19,20,21,22). It remains uncertain whether the same is true of women with isolated PCO. Many of the studies attempting to address this question included oligoovulatory women, preventing the extrapolation of their findings to those with regular menses (6,7,8,10,11). We identified five studies comparing hormonal and metabolic parameters in ovulatory women of reproductive age with PCO with ovulatory women with normal ovaries (23,24,25,26,27). Of these, most found no significant differences in baseline mean gonadotropins (23,24,25,26), androgens (23,24,25,26), lipids (24,26), insulin (23,24,25,26), or glucose (23,24). Some subtle differences were reported in the women with PCO, including a higher proportion with an androgen level above the upper limit of normal as determined by the control group (25) and a larger proportion with high-density lipoprotein (HDL) levels under 35 mg/dl and evidence of insulin resistance (26), but these were not statistically significant. These studies were all limited in their power by a small sample size, including only 10–26 ovulatory women with PCO and 11–26 normal controls.
In contrast to these findings, the largest study, incorporating 39 ovulatory women with PCO and 28 controls, found higher mean T, free T, dehydroepiandrostenedione sulfate (DHEAS), and lower SHBG in women with PCO (27). They also found higher fasting insulin and homeostasis model assessment for insulin resistance (HOMA-IR) in a smaller subset. The mean age of women with PCO was nearly 3 yr older than those without, and age was adjusted for only in the comparison for DHEAS. Additionally, the recruitment methods are not described. This was the only study to apply the Rotterdam criteria for PCO in addition to the Adams criteria, with no significant changes to their findings.
Thus, it remains to be determined on a population basis whether PCO, as defined in Rotterdam, serves as a marker of occult hyperandrogenism or metabolic dysfunction. We hypothesize the application of the new definition will increase the prevalence of PCO among ovulatory women, just as the Rotterdam criteria have increased the prevalence of PCOS (28) and ultimately will provide little insight into the metabolic or hormonal milieu of these women.
Subjects and Methods
Participants
This study includes 262 Caucasian women aged 25–45 yr, enrolled in the OVA study, an ongoing, community-based study of genetic and environmental factors that contribute to ovarian aging in women of diverse race/ethnicity (NIH RO1-HD044876). Inclusion required regular menses at 22- to 35-d intervals. Exclusion criteria included previous ovarian surgery or hysterectomy, use of estrogen or progestin-containing medication within 3 months, pregnancy, lactation, and significant medical or psychiatric illness including diabetes mellitus.
OVA study participants were recruited from potentially eligible female members of Kaiser Permanente Northern California (KP), an integrated healthcare delivery system that provides medical care to over 30% of the population of Northern California. The sociodemographic and health-related characteristics of the KP membership are generally representative of the population of Northern California, as determined by the population-based California Health Interview Study, particularly if the comparison is limited only to those covered by health insurance (29).
Age-eligible KP members of the ethnicities included in the OVA study (African-American, Caucasian, Chinese, and Latina), and living near the research clinic, were identified based on surname and/or zip code. Medical records were screened for diagnostic codes for disorders that would exclude them from participation. Separate data sets were then created according to presumed race/ethnicity and randomly ordered. Successive waves of recruitment letters were mailed, followed by recruitment telephone calls, until the desired number of participants in each race/ethnic group was achieved. The current analysis is based on Caucasian women only because enrollment of that group is complete. Caucasian race was confirmed by self-report at enrollment.
Of the 2121 letters mailed to women presumed to be Caucasian, 559 (26.4%) completed the eligibility screening telephone call. Of these, 130 (23.4%) were found to ineligible before enrollment, and 148 (26.5%) refused further participation, leaving a total of 281 women who enrolled in the cohort and completed the baseline assessment. For this analysis, an additional 17 women were excluded due to inadequate transvaginal ultrasound data, and two were excluded for a menstrual cycle length of less than 22 d or more than 35 d, leaving 262 women in the cohort. Institutional Review Board approval was obtained both from Kaiser Permanente and University of California San Francisco, and all subjects provided informed consent.
Assessments
All subjects underwent measurements of height, weight, and waist and hip circumference. Metabolic assessments included fasting insulin, glucose, and lipids. Hormonal assays performed cycle d 2–4 included FSH, LH, estradiol (E2), estrone (E1), total T, SHBG, anti-Müllerian hormone (AMH), inhibin B, and DHEAS. These parameters were selected based on the previous literature. HOMA-IR (30) and free androgen index (FAI) (31) were calculated.
Participants underwent transvaginal ultrasound assessment of AFC and ovarian volumes, performed between menstrual cycle d 2 and 4. Using a Shimadzu SDU-450XL machine, with a variable 4- to 8-mHz vaginal transducer, the transverse, longitudinal, and anteroposterior diameters of each ovary were measured using electronic calipers, allowing detection of follicles 2 mm or greater in diameter. All echo-free structures in the ovaries with a mean diameter (of two dimensions) of 2–9 mm were counted as antral follicles. Larger structures were considered cysts. The volume of each ovary was calculated using the formula for an ellipsoid: (length × height × width × π/6). All examinations were performed by one of two examiners (M.I.C. and M.P.R.). Before initiation of this study, ultrasounds were performed on 50 infertile women by both examiners; correlation between repeated measurements was 0.92.
The presence or absence of PCO was determined separately by volume (>10 cm3 for either ovary) and AFC (12 or greater for either ovary). Cysts of 10 mm or greater may falsely increase the ovarian volume and decrease the AFC (1). Thus, subjects with an AFC of 12 or greater in either ovary were considered to meet criteria (PCO-AFC), regardless of cysts. Subjects with unilateral cysts were considered not to have PCO-AFC if the AFC in each ovary was less than 12. Subjects with bilateral cysts were excluded from PCO-AFC analyses if the AFC was less than 12 in each ovary; this was found in five participants.
For PCO by volume (PCO-VOL), subjects were classified as negative when both ovaries had volumes of 10 cm3 or less, regardless of cysts. When a unilateral cyst was found, the other ovary used for classification. All subjects with bilateral cysts had ovarian volumes <10 cm3, and thus were classified as negative for PCO-VOL.
Assays
Serum hormonal assays were performed in the Central Ligand Assay Satellite Services (CLASS) Laboratory at the University of Michigan. FSH and LH were measured with standardized two-site chemiluminescence immunoassays. Assay sensitivity was 0.07 IU/liter, intraassay coefficients of variation (CV) were 1.9–2.1%, and interassay CV were 5.2–6.8%.
E1 and inhibin B were assayed using commercially available ELISA from Diagnostic Systems Laboratories (Webster City, TX). E1 sensitivity was 10 pg/ml, intraassay CV 4.8–9.9%, and interassay CV 7.7–13.4%. Inhibin B sensitivity was 7 pg/ml, intraassay CV 3.3–7.2%, and interassay CV 7.8–17%.
E2, DHEAS, SHBG, and T were assayed with an automated chemiluminescent assay using the Bayer Diagnostics ACS:180 (Bayer Diagnostics Corp., Tarrytown, NY). E2 sensitivity was 1 pg/ml; intra- and interassay CV were 6.5–6.9 and 13.6–16.1%, respectively. DHEAS sensitivity was 1.9 μg/dl, intraassay CV 10.9–11.2%, and interassay CV 16.4–19.0%. SHBG sensitivity was 1.95 nmol/liter, intraassay CV 6.4–7.0%, and interassay CV 6.9–8.9%. The T assay was modified to improve precision in the lower range by increasing the sample volume while evaluating the effect on volumes of subsequent reagents. The limit of detection of 5.15 ng/dl, intraassay CV 2.9–4.0%, and interassay CV 6.3–6.6%.
AMH was assayed using the commercially available ELISA from Beckman Coulter (Marseille, France), which uses a two-site sandwich-type immunoassay. Assay sensitivity was 0.12 pmol/liter, the intraassay CV 5.6%, and the interassay CV 15.3%.
Glucose, insulin, and lipid assays were performed by Quest Diagnostics (San Jose, CA). Fasting glucose was assayed by the glucose oxidase method, with CV of 1.25–2.00%. The Siemens Immulite (Tarrytown, NY) immunochemiluminometric assay was used for insulin, with CV of 3.64–6.64% and sensitivity of 2.0 μIU/ml. Total and HDL cholesterol and triglycerides were assayed using enzymatic methods, with CV of 1.32–1.92, 1.15–2.02, and 1.99–3.45%, respectively.
Statistical analysis
Demographic variables in the study sample were compared with data on women members of KP aged 20–44 yr residing in the same geographical location using χ2 statistics.
All subsequent analyses were performed separately for PCO-AFC and PCO-VOL. Age was categorized into 5-yr strata. The Cochran-Armitage test was used to evaluate for a trend in the prevalence of PCO across the age strata. Generalized linear models were generated to determine whether tobacco use related to AFC or mean ovarian volume, adjusting for age.
To determine whether hormonal and metabolic parameters differed between those with and without PCO-AFC, linear regression analyses were performed, adjusted for age. From these models, adjusted least square means (LSM) of the hormonal and metabolic parameters among those with and without PCO-AFC were calculated. Next, multivariate linear regression techniques were used to control for both age and body mass index (BMI) while comparing those hormonal and metabolic parameters that differed significantly between those with and without PCO-AFC in the original regressions.
These analyses were then repeated comparing those with and without PCO-VOL. To determine whether the effect of volume was due to an increased number of follicles, multivariate linear regression analyses adjusting for AFC and age were performed for outcomes that were significantly associated with PCO-VOL.
A sensitivity analysis was performed, excluding subjects with an E2 higher than 200 pg/ml, all of whom had a follicle or cyst larger than 10 mm, to assure that significant findings were not driven by those not in a normal early follicular phase. To address multiple comparisons, stepwise selection was used to generate multivariate logistic regression models to predict PCO-AFC and PCO-VOL. All hormonal and metabolic parameters were evaluated for inclusion using a significance level of 0.05. Efficiency was enhanced at every step using the Newton-Rhapson estimation technique.
Results
Baseline sample characteristics are shown in Table 1. In comparison with white women KP members, ages 20–44 yr, residing in the same geographic area from which the sample was recruited, the OVA participants were less likely to be married or living with a partner (44.3 vs. 67.5%, χ2 = 14.8; P = 0.0001) (29). They were similar in terms of tobacco use, income, education, and employment status (data not shown).
Table 1.
Baseline sample characteristics
| n | % | |
|---|---|---|
| Age (yr) | ||
| 25–30 | 52 | 19.9 |
| 31–35 | 88 | 33.6 |
| 36–40 | 79 | 30.2 |
| 41–45 | 43 | 16.4 |
| Marital status | ||
| Married, living like married | 116 | 44.3 |
| Separated or divorced | 24 | 9.2 |
| Never married | 122 | 46.6 |
| Highest educational level attained | ||
| HS grad/GED | 6 | 2.3 |
| Some college or vocational/technical school | 33 | 12.6 |
| College graduate (BS or BA) | 130 | 49.6 |
| Graduate or professional degree | 93 | 35.5 |
| Employment status | ||
| Working full time | 204 | 78.03 |
| Working part time | 33 | 12.5 |
| Keeping house/raising children | 10 | 3.79 |
| Unemployed or laid off | 15 | 5.7 |
| Annual family income | ||
| <$25,000 | 11 | 4.2 |
| $25,000 to <$50,000 | 83 | 31.7 |
| $50,000 to <$100,000 | 88 | 33.6 |
| $100,000 to <$199,000 | 71 | 27.1 |
| $200,000 or greater | 8 | 3.1 |
| Tobacco use | ||
| Current | 35 | 13.4 |
| Past | 79 | 30.2 |
| Never | 148 | 56.5 |
| Obstetric and gynecological history | ||
| Menstrual cycle length (d) | ||
| 22–24 | 7 | 2.7 |
| 25–27 | 57 | 21.8 |
| 28–32 | 184 | 70.2 |
| 33–35 | 14 | 5.3 |
| Previous oral contraceptive use | ||
| None | 52 | 19.9 |
| <1 yr | 40 | 15.3 |
| 1–5 yr | 91 | 34.7 |
| 5–10 yr | 42 | 16 |
| >10 yr | 37 | 14.1 |
| Past pregnancy | 94 | 35.6 |
| No. of full-term pregnancies | ||
| None | 221 | 84.4 |
| One | 14 | 5.3 |
| Two or three | 26 | 9.9 |
| Four or more | 1 | 0.4 |
| Past infertility diagnosis | 6 | 2.6 |
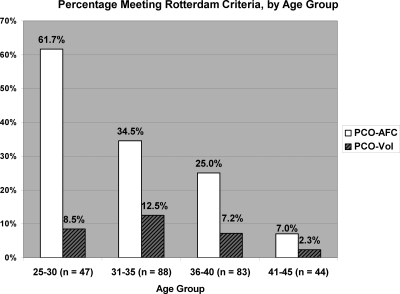
Of 262 subjects, three had bilateral cysts that did not prevent classification, and 57 (21.8%) had a unilateral cyst. Thirty-two percent of all subjects had PCO-AFC, and 8.4% PCO-VOL. All patients with PCO-VOL also had PCO-AFC. In Fig. 1, the prevalence of PCO-AFC and PCO-VOL, by age group, is noted. The proportion of women with PCO-AFC significantly decreased with increasing age (Z = 5.63; P < 0.001). However, there was no statistically significant trend in the proportion with PCO-VOL (Z = 1.47; P = 0.14). Tobacco use was unrelated to AFC (P = 0.15) or mean ovarian volume (P = 0.43) and, thus, was not included in the later models.
Figure 1.
Percentage of subjects meeting each portion of the Rotterdam Criteria, by age group.
In Table 2 are the age-adjusted LSM values of hormonal and metabolic parameters among those with and without PCO-AFC. BMI was unrelated to ovarian volume or AFC as continuous variables. Differences in hormone concentrations (higher E1, AMH, T, and DHEAS and lower FSH) remained statistically significant when adjusting for both BMI and age, but the differences in FAI and waist circumference did not. Exclusion of those with E2 higher than 200 pg/ml did not significantly change these findings. In the final stepwise selection model, AMH, DHEAS, and BMI were retained as independent predictors of PCO-AFC.
Table 2.
Age-adjusted LSM values among women with and without PCO-AFC, with differences and 95% confidence intervals (CI)
| LSM
|
Difference | 95% CI | P value | ||
|---|---|---|---|---|---|
| PCO-AFC (n = 82) | Without PCO-AFC (n = 175) | ||||
| Hormonal | |||||
| FSH (IU/liter) | 6.23 | 7.35 | −1.12 | −1.99 to −0.25 | 0.01 |
| LH (IU/liter) | 5.88 | 5.55 | 0.33 | −0.49–1.16 | 0.43 |
| E2 (pg/ml) | 46.5 | 46.2 | 0.3 | −9.9–10.4 | 0.96 |
| E1 (pg/ml) | 66.6 | 57.7 | 8.9 | 3.5–14.2 | 0.001 |
| AMH (pmol/liter) | 45.3 | 23.3 | 22.0 | 16.8–27.2 | <0.001 |
| AMH to AFC ratio | 1.80 | 2.01 | −0.21 | −0.51–0.09 | 0.17 |
| Inhibin B (pg/ml) | 56.0 | 54.6 | 1.3 | −6.8–9.5 | 0.75 |
| T (ng/dl) | 55.7 | 50.5 | 5.2 | 1.1–9.3 | 0.01 |
| SHBG (nmol/liter) | 47.2 | 51.4 | −4.2 | −9.2–0.9 | 0.11 |
| FAI | 4.96 | 3.95 | 1.01 | 0.25–1.77 | 0.01 |
| DHEAS (μg/dl) | 245 | 207 | 37 | 6–68 | 0.02 |
| Metabolic | |||||
| BMI (kg/m2) | 25.6 | 24.0 | 1.6 | 0.04–3.1 | 0.04 |
| Waist circumference (cm) | 79.3 | 76.1 | 3.2 | 0.1–6.3 | 0.045 |
| Fasting glucose (mg/dl) | 86.9 | 86.3 | 0.6 | −1.6–2.8 | 0.59 |
| Insulin (μIU/ml) | 5.08 | 4.23 | 0.84 | −0.36–2.05 | 0.17 |
| HOMA-IR | 1.16 | 0.91 | 0.24 | −0.05–0.54 | 0.10 |
| Total cholesterol (mg/dl) | 173 | 173 | 0 | −8–8 | 1.00 |
| HDL cholesterol (mg/dl) | 62.9 | 64.3 | −1.4 | −5.4–2.7 | 0.50 |
| LDL cholesterol (mg/dl) | 93.0 | 93.5 | −0.5 | −7.5–6.4 | 0.88 |
| VLDL cholesterol (mg/dl) | 17.1 | 15.0 | 2.1 | −0.3–4.5 | 0.09 |
| Total cholesterol to HDL ratio | 2.89 | 2.80 | 0.10 | −0.12–0.31 | 0.38 |
| Triglycerides (mg/dl) | 85 | 77 | 8 | −6–21 | 0.26 |
Subjects were those who also have PCO-VOL included. To convert to SI units, multiply E2 by 3.67, E1 by 3.7, T by 0.0347, DHEAS by 0.026, glucose by 0.056, insulin by 6.945, triglycerides by 0.011, and total, HDL, low-density lipoprotein (LDL), and very-low-density lipoprotein (VLDL) cholesterol by 0.0259.
In Table 3 are the age-adjusted LSM values of hormonal and metabolic parameters among those with and without PCO-VOL. Women with PCO-VOL had higher AMH and FAI than those with smaller ovaries but no other statistically significant hormonal or metabolic differences. These differences remained significant when adjusting for both BMI and age. When AFC was added to the model predicting AMH, PCO-VOL was no longer a statistically significant predictor. Exclusion of those with E2 higher than 200 pg/ml did not significantly change these findings. Only AMH was retained in the final stepwise selection model.
Table 3.
Age-adjusted LSM values among women with and without PCO-VOL, with differences and 95% confidence intervals (CI)
| LSM
|
Difference | 95% CI | P value | ||
|---|---|---|---|---|---|
| PCO-VOL (n = 22) | Without PCO-VOL* (n = 240) | ||||
| Hormonal | |||||
| FSH (IU/liter) | 6.18 | 7.08 | −0.91 | −2.29–0.47 | 0.20 |
| LH (IU/liter) | 5.55 | 5.79 | −0.24 | −1.90–1.42 | 0.77 |
| E2 (pg/ml) | 45.1 | 49.9 | −4.8 | −27.8–18.2 | 0.68 |
| Estrone (pg/ml) | 62.8 | 61.7 | 1.1 | −9.7–11.9 | 0.85 |
| AMH (pmol/liter) | 51.9 | 27.9 | 24.1 | 15.4–32.8 | <0.001 |
| AMH to AFC ratio | 1.75 | 1.94 | −0.19 | −0.68–0.29 | 0.44 |
| Inhibin B (pg/ml) | 66.1 | 53.6 | 12.4 | −0.3–25.1 | 0.06 |
| T (ng/dl) | 55.2 | 52.1 | 3.1 | −3.44–9.74 | 0.35 |
| SHBG (nmol/liter) | 49.1 | 50.7 | −1.6 | −9.8–6.6 | 0.70 |
| FAI | 5.68 | 4.13 | 1.55 | 0.36–2.74 | 0.01 |
| DHEAS (μg/dl) | 192 | 222 | −29 | −78–19.3 | 0.24 |
| Metabolic | |||||
| BMI (kg/m2) | 25.4 | 24.5 | 1.0 | −1.5–3.5 | 0.44 |
| Waist circumference (cm) | 77.8 | 77.2 | 0.6 | −4.4–5.6 | 0.81 |
| Fasting glucose (mg/dl) | 88.8 | 86.2 | 2.7 | −0.8–6.2 | 0.14 |
| Insulin (μIU/ml) | 5.38 | 4.51 | 0.87 | −1.12–2.86 | 0.39 |
| HOMA–IR | 1.26 | 0.98 | 0.28 | −0.19–0.76 | 0.24 |
| Total cholesterol (mg/dl) | 185 | 172 | 12 | −1–25 | 0.06 |
| HDL cholesterol (mg/dl) | 65.6 | 63.9 | 1.6 | −4.9–8.1 | 0.62 |
| LDL cholesterol (mg/dl) | 101.2 | 92.5 | 8.7 | −2.3–19.7 | 0.12 |
| VLDL cholesterol (mg/dl) | 17.8 | 15.4 | 2.4 | −1.4–6.2 | 0.21 |
| Total cholesterol to HDL ratio | 3.02 | 2.82 | 0.20 | −0.17–0.56 | 0.29 |
| Triglycerides (mg/dl) | 89.5 | 79.9 | 9.6 | −133–32.6 | 0.41 |
Subjects include thoses with PCO-AFC who did not meet the PCO-VOL criterion. To convert to SI units, multiply E2 by 3.67, E1 by 3.7, T by 0.0347, DHEAS by 0.026, glucose by 0.056, insulin by 6.945, triglycerides by 0.011, and total, HDL, low-density lipoprotein (LDL), and very-low-density lipoprotein (VLDL) cholesterol by 0.0259.
Discussion
This study is the largest to date assessing the prevalence of PCO in normal ovulatory women. We demonstrate that PCO by the Rotterdam definition are common and are not indicative of all the metabolic derangements seen in PCOS. At 31.9%, the prevalence of PCO by the Rotterdam definition among women aged 25–45 yr is higher than that reported for the Adams definition (6,7,8,9,10,11) and comparable to that recently reported for the Rotterdam definition (18) when age differences between the study populations are considered. Advances in ultrasound technology allowing accurate detection of small antral follicles may have contributed to the increase in prevalence. Furthermore, we demonstrate the prevalence of PCO by the Rotterdam AFC criterion is significantly impacted by age, consistent with data indicating a fall in AFC with age among both ovulatory (12,13,14,15,16) and PCOS (17) women. This is consistent with longitudinal data showing half of women with PCO at a mean age of 30 no longer display this finding 8 yr later (32).
Women meeting the AFC portion of the Rotterdam criteria differ from their peers hormonally, with higher AMH, T, and E1. Because these are all products of small ovarian follicles up to the antral stage (33), it is not surprising that women with more antral follicles would produce more of each of these hormones. Similarly, it is not surprising that women with more antral follicles would display lower FSH, also a marker of ovarian reserve.
Our finding of increased serum T is consistent with the largest previous study on this topic (27) and contrasts with the smaller studies (23,24,25,26). These smaller studies likely lacked the statistical power to detect a difference of approximately 10%. Importantly, we found that women with T above the 95th percentile for the entire group are distributed as expected, with four meeting the PCO-AFC criterion and eight not. These findings suggest androgens in women with PCO by AFC may fall in the higher portion of the normal range but show hyperandrogenemia is not common in this group. Serum T correlates with AFC in women with PCOS (34); our data would suggest a similar relationship in ovulatory women.
Our finding of increased AMH in women with PCO-AFC is consistent with data demonstrating AMH to be an appropriate marker of ovarian reserve, correlating with AFC in normal women (35,36) and in women with PCOS (37). AMH has been suggested to play a role in the impaired follicular selection seen in anovulatory women with PCOS and has been proposed as a diagnostic criterion for PCOS in lieu of AFC (37). However, Laven et al. (35) reported higher levels of AMH in anovulatory women with PCO than in anovulatory women without PCO, highlighting the relationship between AMH and AFC independent of ovulatory status. They reported a mean AMH to follicle ratio of 2.3 pmol/liter in women with PCOS and 1.07 pmol/liter in 42 control women aged 20–26 yr; this contrasts with our finding of an AMH to follicle ratio of 1.80 pmol/liter in women with PCO-AFC alone and 2.01 pmol/liter in women without. We would argue that in ovulatory women, AMH, like AFC, serves as a marker of ovarian reserve and fails to differentiate women with other features of PCOS.
Despite differences in androgens and AMH, ovulatory women with PCO-AFC lack the metabolic profile of women with PCOS. There is a small but statistically significant increase in BMI among these women; however, we found no significant differences in other metabolic measures. Our findings are consistent with a previous study that found a greater BMI in women with PCO alone than in either women with normal ovaries or those with classic PCOS; as in our study, insulin and glucose were similar among all women (24). Moreover, the majority of research in this area has failed to find metabolic changes among women with PCO alone (10,25,26,27,38). We did not perform an oral glucose tolerance test, and it is possible that this would have uncovered metabolic differences associated with PCO. However, women with PCO alone have lower fasting insulin (25,26) and higher insulin sensitivity (10) than women with PCOS. Thus, PCO alone cannot be considered a marker for metabolic dysfunction or cardiovascular risk. Ovulatory women with PCO do not require metabolic screening beyond that dictated by their age and clinical risk factors.
An ovarian volume of more than 10 cm3 is less common than an AFC of 12 or higher and could represent the upper limit of normal. Although ovarian volume decreases with age (13), the proportion of women with PCO-VOL does not trend downward with increasing age. As with the AFC criterion, these women show higher serum concentrations of AMH, which is explained by their increased AFC. Women with PCO-VOL do not differ significantly from their peers in any metabolic parameter.
Similarly, other characterizations of PCO fail to predict metabolic disturbance. Legro et al. (23) found an increased prevalence of PCO by the Adams criteria in women with PCOS compared with controls but also demonstrated that PCO does not predict insulin, glucose, or androgens in women with PCOS. Women with PCO alone, as defined by the Adams criteria (25) or a volume-based criterion (38) have lower androgens than those with PCOS. They also display lower mean AFC than those with PCOS (39), so it is possible that use of a higher threshold for AFC or solely counting follicles 2–5 mm in size might be more specific for PCOS (2,40).
This study included Caucasians only. Although this limits the applicability of our findings to other ethnicities, we are continuing to enroll participants of African-American, Latina, and Asian ancestries and, thus, in future work will assess for consistency among these groups. Additionally, study subjects were not evaluated for clinical hyperandrogenism because this was not the focus of the original study. Thus, a small number of women meeting Rotterdam criteria for PCOS, despite ovulatory menses, may have been included. However, inclusion of these women in the PCO groups would have biased our findings toward greater differences in metabolic parameters, because women with ovulatory, hyperandrogenic PCOS have greater insulin, HOMA-IR, and triglyceride levels than age- and BMI-matched controls (41).
If PCO alone is not associated with metabolic risk, does this finding confer additional metabolic risk to non-hyperandrogenic anovulatory women or to ovulatory women with hyperandrogenism? A large body of evidence, reviewed by Moran and Teede (42), suggests women displaying the Rotterdam PCOS phenotypes consisting of PCO and either hyperandrogenism or anovulation show less severe metabolic dysfunction than those with both hyperandrogenism and anovulation and in many cases do not differ from normal controls. As with PCO, serum androgens and menstrual irregularity decrease with age in women with PCOS (43); thus, these criteria may introduce age bias into the diagnosis.
We demonstrate herein that PCO by the Rotterdam definition is an age-dependent, normal finding among ovulatory women, with no significance in predicting cardiovascular risk. In isolation, PCO should not be considered pathology. The high prevalence of PCO among normal women raises concerns about its specificity as a diagnostic criterion for PCOS.
Footnotes
This work was supported by Grant R01HD044876 from the National Institute of Child Health and Human Development/National Institute on Aging, 2-T32-HD040135-06 from the National Institutes of Health (NIH), and University of California, San Francisco, Clinical and Translational Science Institute Grant UL1 RR024131 from the NIH/National Center for Research Resources. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Disclosure Summary: The authors have nothing to disclose.
First Published Online August 18, 2010
Abbreviations: AFC, Antral follicle count; AMH, anti-Müllerian hormone; DHEAS, dehydroepiandrostenedione sulfate; E1, estrone; E2, estradiol; FAI, free androgen index; HDL, high-density lipoprotein; HOMA-IR, homeostasis model assessment for insulin resistance; LSM, least square means; PCO, polycystic ovary; PCOS, PCO syndrome; PCO-VOL, PCO by volume; T, testosterone.
References
- Balen AH, Laven JS, Tan SL, Dewailly D 2003 Ultrasound assessment of the polycystic ovary: international consensus definitions. Hum Reprod Update 9:505–514 [DOI] [PubMed] [Google Scholar]
- Jonard S, Robert Y, Cortet-Rudelli C, Pigny P, Decanter C, Dewailly D 2003 Ultrasound examination of polycystic ovaries: is it worth counting the follicles? Hum Reprod 18:598–603 [DOI] [PubMed] [Google Scholar]
- Pache TD, Wladimiroff JW, Hop WC, Fauser BC 1992 How to discriminate between normal and polycystic ovaries: transvaginal US study. Radiology 183:421–423 [DOI] [PubMed] [Google Scholar]
- van Santbrink EJ, Hop WC, Fauser BC 1997 Classification of normogonadotropic infertility: polycystic ovaries diagnosed by ultrasound versus endocrine characteristics of polycystic ovary syndrome. Fertil Steril 67:452–458 [DOI] [PubMed] [Google Scholar]
- Adams J, Polson DW, Franks S 1986 Prevalence of polycystic ovaries in women with anovulation and idiopathic hirsutism. Br Med J (Clin Res Ed) 293:355–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton RN, Ogden V, Hodgkinson J, Worswick L, Rodin DA, Dyer S, Meade TW 1992 How common are polycystic ovaries in normal women and what is their significance for the fertility of the population? Clin Endocrinol (Oxf) 37:127–134 [DOI] [PubMed] [Google Scholar]
- Polson DW, Adams J, Wadsworth J, Franks S 1988 Polycystic ovaries: a common finding in normal women. Lancet 1:870–872 [DOI] [PubMed] [Google Scholar]
- Farquhar CM, Birdsall M, Manning P, Mitchell JM, France JT 1994 The prevalence of polycystic ovaries on ultrasound scanning in a population of randomly selected women. Aust NZ J Obstet Gynaecol 34:67–72 [DOI] [PubMed] [Google Scholar]
- Abdel Gadir A, Khatim MS, Mowafi RS, Alnaser HM, Muharib NS, Shaw RW 1992 Implications of ultrasonically diagnosed polycystic ovaries. I. Correlations with basal hormonal profiles. Hum Reprod 7:453–457 [DOI] [PubMed] [Google Scholar]
- Michelmore KF, Balen AH, Dunger DB, Vessey MP 1999 Polycystic ovaries and associated clinical and biochemical features in young women. Clin Endocrinol (Oxf) 51:779–786 [DOI] [PubMed] [Google Scholar]
- Koivunen R, Laatikainen T, Tomás C, Huhtaniemi I, Tapanainen J, Martikainen H 1999 The prevalence of polycystic ovaries in healthy women. Acta Obstet Gynecol Scand 78:137–141 [PubMed] [Google Scholar]
- Ruess ML, Kline J, Santos R, Levin B, Timor-Tritsch I 1996 Age and the ovarian follicle pool assessed with transvaginal ultrasonography. Am J Obstet Gynecol 174:624–627 [DOI] [PubMed] [Google Scholar]
- Erdem A, Erdem M, Biberoglu K, Hayit O, Arslan M, Gursoy R 2002 Age-related changes in ovarian volume, antral follicle counts and basal FSH in women with normal reproductive health. J Reprod Med 47:835–839 [PubMed] [Google Scholar]
- Kline J, Kinney A, Kelly A, Reuss ML, Levin B 2005 Predictors of antral follicle count during the reproductive years. Hum Reprod 20:2179–2189 [DOI] [PubMed] [Google Scholar]
- Huang FJ, Chang SY, Tsai MY, Kung FT, Wu JF, Chang HW 2001 Determination of the efficiency of controlled ovarian hyperstimulation in the gonadotropin-releasing hormone agonist-suppression cycle using the initial follicle count during gonadotropin stimulation. J Assist Reprod Genet 18:91–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffer GJ, Broekmans FJ, Looman CW, Blankenstein M, Fauser BC, teJong FH, teVelde ER 2003 The number of antral follicles in normal women with proven fertility is the best reflection of reproductive age. Hum Reprod 18:700–706 [DOI] [PubMed] [Google Scholar]
- Piltonen T, Morin-Papunen L, Koivunen R, Perheentupa A, Ruokonen A, Tapanainen JS 2005 Serum anti-Mullerian hormone levels remain high until late reproductive age and decrease during metformin therapy in women with polycystic ovary syndrome. Hum Reprod 20:1820–1826 [DOI] [PubMed] [Google Scholar]
- Duijkers IJ, Klipping C 2010 Polycystic ovaries, as defined by the 2003 Rotterdam consensus criteria, are found to be very common in young healthy women. Gynecol Endocrinol 26:152–160 [DOI] [PubMed] [Google Scholar]
- Shroff R, Kerchner A, Maifeld M, Van Beek EJ, Jagasia D, Dokras A 2007 Young obese women with polycystic ovary syndrome have evidence of early coronary atherosclerosis. J Clin Endocrinol Metab 92:4609–4614 [DOI] [PubMed] [Google Scholar]
- Marcondes JA, Hayashida SA, Barcellos CR, Rocha MP, Maciel GA, Baracat EC 2007 Metabolic syndrome in women with polycystic ovary syndrome: prevalence, characteristics and predictors. Arq Bras Endocrinol Metabol 51:972–979 [DOI] [PubMed] [Google Scholar]
- Ehrmann DA, Liljenquist DR, Kasza K, Azziz R, Legro RS, Ghazzi MN 2006 Prevalence and predictors of the metabolic syndrome in women with polycystic ovary syndrome. J Clin Endocrinol Metab 91:48–53 [DOI] [PubMed] [Google Scholar]
- Glueck CJ, Papanna R, Wang P, Goldenberg N, Sieve-Smith L 2003 Incidence and treatment of metabolic syndrome in newly referred women with confirmed polycystic ovarian syndrome. Metabolism 52:908–915 [DOI] [PubMed] [Google Scholar]
- Legro RS, Chiu P, Kunselman AR, Bentley CM, Dodson WC, Dunaif A 2005 Polycystic ovaries are common in women with hyperandrogenic chronic anovulation but do not predict metabolic or reproductive phenotype. J Clin Endocrinol Metab 90:2571–2579 [DOI] [PubMed] [Google Scholar]
- Norman RJ, Hague WM, Masters SC, Wang XJ 1995 Subjects with polycystic ovaries without hyperandrogenaemia exhibit similar disturbances in insulin and lipid profiles as those with polycystic ovary syndrome. Hum Reprod 10:2258–2261 [DOI] [PubMed] [Google Scholar]
- Carmina E, Wong L, Chang L, Paulson RJ, Sauer MV, Stanczyk FZ, Lobo RA 1997 Endocrine abnormalities in ovulatory women with polycystic ovaries on ultrasound. Hum Reprod 12:905–909 [DOI] [PubMed] [Google Scholar]
- Chang PL, Lindheim SR, Lowre C, Ferin M, Gonzalez F, Berglund L, Carmina E, Sauer MV, Lobo RA 2000 Normal ovulatory women with polycystic ovaries have hyperandrogenic pituitary-ovarian responses to gonadotropin-releasing hormone-agonist testing. J Clin Endocrinol Metab 85:995–1000 [DOI] [PubMed] [Google Scholar]
- Adams JM, Taylor AE, Crowley Jr WF, Hall JE 2004 Polycystic ovarian morphology with regular ovulatory cycles: insights into the pathophysiology of polycystic ovarian syndrome. J Clin Endocrinol Metab 89:4343–4350 [DOI] [PubMed] [Google Scholar]
- Broekmans FJ, Knauff EA, Valkenburg O, Laven JS, Eijkemans MJ, Fauser BC 2006 PCOS according to the Rotterdam consensus criteria: change in prevalence among WHO-II anovulation and association with metabolic factors. BJOG 113:1210–1217 [DOI] [PubMed] [Google Scholar]
- Gordon NP 2006 How does the adult Kaiser Permanente membership in Northern California compare with the larger community? Oakland, CA: Kaiser Permanente Division of Research [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC 1985 Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419 [DOI] [PubMed] [Google Scholar]
- Södergård R, Bäckström T, Shanbhag V, Carstensen H 1982 Calculation of free and bound fractions of testosterone and estradiol-17β to human plasma proteins at body temperature. J Steroid Biochem 16:801–810 [DOI] [PubMed] [Google Scholar]
- Murphy MK, Hall JE, Adams JM, Lee H, Welt CK 2006 Polycystic ovarian morphology in normal women does not predict the development of polycystic ovary syndrome. J Clin Endocrinol Metab 91:3878–3884 [DOI] [PubMed] [Google Scholar]
- Weenen C, Laven JS, Von Bergh AR, Cranfield M, Groome NP, Visser JA, Kramer P, Fauser BC, Themmen AP 2004 Anti-Mullerian hormone expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitment. Mol Hum Reprod 10:77–83 [DOI] [PubMed] [Google Scholar]
- Chen MJ, Yang WS, Chen CL, Wu MY, Yang YS, Ho HN 2008 The relationship between anti-Mullerian hormone, androgen and insulin resistance on the number of antral follicles in women with polycystic ovary syndrome. Hum Reprod 23:952–957 [DOI] [PubMed] [Google Scholar]
- Laven JS, Mulders AG, Visser JA, Themmen AP, De Jong FH, Fauser BC 2004 Anti-Mullerian hormone serum concentrations in normoovulatory and anovulatory women of reproductive age. J Clin Endocrinol Metab 89:318–323 [DOI] [PubMed] [Google Scholar]
- Su HI, Sammel MD, Freeman EW, Lin H, DeBlasis T, Gracia CR 2008 Body size affects measures of ovarian reserve in late reproductive age women. Menopause 15:857–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigny P, Jonard S, Robert Y, Dewailly D 2006 Serum anti-Mullerian hormone as a surrogate for antral follicle count for definition of the polycystic ovary syndrome. J Clin Endocrinol Metab 91:941–945 [DOI] [PubMed] [Google Scholar]
- Mortensen M, Rosenfield RL, Littlejohn E 2006 Functional significance of polycystic-size ovaries in healthy adolescents. J Clin Endocrinol Metab 91:3786–3790 [DOI] [PubMed] [Google Scholar]
- Ng EH, Chan CC, Ho PC 2006 Are there differences in ultrasound parameters between Chinese women with polycystic ovaries only and with polycystic ovary syndrome? Eur J Obstet Gynecol Reprod Biol 125:92–98 [DOI] [PubMed] [Google Scholar]
- Dewailly D, Catteau-Jonard S, Reyss AC, Maunoury-Lefebvre C, Poncelet E, Pigny P 2007 The excess in 2–5 mm follicles seen at ovarian ultrasonography is tightly associated to the follicular arrest of the polycystic ovary syndrome. Hum Reprod 22:1562–1566 [DOI] [PubMed] [Google Scholar]
- Rizzo M, Berneis K, Hersberger M, Pepe I, Di Fede G, Rini GB, Spinas GA, Carmina E 2009 Milder forms of atherogenic dyslipidemia in ovulatory versus anovulatory polycystic ovary syndrome phenotype. Hum Reprod 24:2286–2292 [DOI] [PubMed] [Google Scholar]
- Moran L, Teede H 2009 Metabolic features of the reproductive phenotypes of polycystic ovary syndrome. Hum Reprod Update 15:477–488 [DOI] [PubMed] [Google Scholar]
- Bili H, Laven J, Imani B, Eijkemans MJ, Fauser BC 2001 Age-related differences in features associated with polycystic ovary syndrome in normogonadotrophic oligo-amenorrhoeic infertile women of reproductive years. Eur J Endocrinol 145:749–755 [DOI] [PubMed] [Google Scholar]