Abstract
Context: Insulin resistance is a major risk factor for the development of type 2 diabetes in Pima Indians, a population with the highest prevalence of type 2 diabetes mellitus in the world. Their Mexican counterpart, living a traditional lifestyle in the mountains of Sonora, have at least 5 times less diabetes than the U.S. Pima Indians.
Objective: We evaluated whether Mexican Pima Indians had lower insulin resistance than U.S. Pima Indians.
Design and Patients: We compared fasting insulin and homeostasis model assessment for insulin resistance (HOMA-IR) in 194 Mexican Pima Indians (100 females, 94 males) and 449 U.S. Pima Indians (246 females, 203 males) with normal glucose tolerance from a cross-sectional study. Adjusted differences of log-transformed outcomes (fasting insulin and HOMA-IR) between groups were evaluated using multiple linear regression models and paired t test in a matched subset.
Results: Unadjusted fasting insulin and HOMA-IR were much lower in the Mexican Pima Indians than in their U.S. counterparts. After adjusting by obesity, age, and sex, mean (95% confidence interval) for fasting insulin was 6.22 (5.34–7.24) vs. 13.56 μU/ml (12.27–14.97) and for HOMA-IR 1.40 (1.20–1.64) vs. 3.07 (2.77–3.40), respectively, for Mexican Pima and U.S. Pima Indians. Results were confirmed in subset matched for age, sex, and body fat.
Conclusion: Our results indicate that Mexican Pima Indians have lower insulin resistance in comparison with their genetically related U.S. counterparts, even after controlling for differences in obesity, age, and sex. This finding underscores the importance of lifestyle factors as protecting factors against insulin resistance in individuals with a high propensity to develop diabetes.
Mexican Pima Indians with normal glucose tolerance are less insulin resistant than their genetically related U.S. counterparts, independent of obesity, age, and sex.
Insulin resistance is a major risk factor in the pathogenesis of type 2 diabetes mellitus. Prospective studies have shown that insulin resistance is an independent predictor of type 2 diabetes (1) including the U.S. Pima Indians (2,3), a population with the highest reported prevalence of type 2 diabetes and obesity in the world (4). A longitudinal study designed to investigate the pathogenesis of type 2 diabetes in this population has characterized independent predictors of diabetes including obesity, insulin resistance, insulin secretory dysfunction, and increased rate of endogenous glucose production (2,5).
Mexican Pima Indians, a population living a traditional lifestyle in the mountains of northwestern Mexico, have a much higher physical activity (6,7) and a diet lower in fat and higher in fiber and complex carbohydrates (6) in comparison with the U.S. Pima. Mexican Pima Indians also have an age- and sex-standardized prevalence of type 2 diabetes at least 5 times lower than that U.S. Pima Indians (6). Differences in type 2 diabetes in these two genetically related populations (8) have been attributed largely to differences in body weight and body fat, probably due to differences in diet and physical activity (6). Although insulin resistance has been widely studied in the U.S. Pima Indians (2,3), information is lacking in Mexican Pima Indians.
The aim of the present work was to investigate whether Mexican Pima Indians have a lower insulin resistance, evaluated by fasting insulin and homeostasis model assessment for insulin resistance (HOMA-IR), than U.S. Pima Indians.
Patients and Methods
Subjects
The Mexican Pima study have been previously described (6,7). Briefly, residents of Maycoba, Sonora, Mexico, and the surrounding areas were invited to participate in a health examination at our research clinic in the village of El Kipor, 10 km east of Maycoba.
Only subjects with normal glucose tolerance were included in this analysis. The Mexican Pima sample consisted of all subjects 20 yr of age and older, who took part in a population-based cross-sectional study aimed at measuring the prevalence of type 2 diabetes and obesity in the year 1996. A total of 224 Mexican Pima Indians of both sexes participated in the study of whom 194 had normal glucose tolerance. The study was approved by the ethics committees of the University of Milwaukee and the Centro de Investigacion en Alimentacion y Desarrollo, Asociacion Civil. All subjects gave written informed consent before participation.
Since 1965, a longitudinal study of type 2 diabetes and its complications has been conducted in the Gila River Indian Community. Approximately every 2 yr, each resident of this community who is at least 5 yr old is invited to participate in examinations (4,6). Based on this ongoing epidemiological study, a sample of 887 U.S. Pima Indians were selected for comparison with the Mexican Pima Indians regarding prevalence of type 2 diabetes and obesity (6). Thus, U.S. Pima participants who were 20 y old or older were selected as having been examined during a similar time period as in the Mexican Pima study (6). For the current analysis, all subjects with normal glucose tolerance (n = 449) from the U.S. Pima Indians sample (n = 887) were included. In addition, a subset of Mexican and U.S. Pima Indians was selected, pair matched on the basis of age, sex, and percent body fat (n = 79 pairs).
Biochemical measures
Oral glucose tolerance tests were performed using a 75-g glucose load after 10–12 h fasting according to World Health Organization recommendations (9). Biochemical measures were in serum for fasting and 2-h after glucose for concentrations of glucose, insulin, triglycerides, and total and high-density lipoprotein (HDL) cholesterol. All measures followed the same protocol as those in the U.S. Pima cohort study; biochemical analyses were conducted at the Phoenix Epidemiology and Clinical Research Branch. Plasma glucose concentrations were measured with an autoanalyzer using a glucose hexokinase (Ciba Corning Express, Norwood, MA). Type 2 diabetes and impaired glucose tolerance were defined according to the World Health Organization criteria (9), but only persons with normal glucose tolerance (fasting plasma glucose <126 mg/dl and 2 h plasma glucose <140 mg/dl) were included in the present analysis. Plasma insulin concentrations were determined using an automated RIA analyzer (Concept 4; INC Biomedicals, Horsham, PA). The response variables were insulin resistance measured by fasting insulin (microunits per milliliter) and HOMA-IR [(fasting Insulin (microunits per milliliter) × fasting glucose (millimoles per liter)]/22.5 (10). Triglycerides and total and HDL cholesterol were measured by enzymatic methods (11,12,13).
Physical measures
Body mass index (BMI) was calculated by dividing weight in kilograms by the square of height in meters. Central obesity was assessed by waist circumference in centimeters. Percent body fat was estimated from bioelectrical impedance (BIA-103; RJL Systems, Detroit, MI) using an equation developed for the U.S. Pima Indians (14). Blood pressure was measured to the nearest 2 mm Hg with a mercury sphygmomanometer (desk model mercury sphygmomanometer, model 100; Liberator Medical Supply, Stuart, FL) in the right arm while the subjects rested in a sitting position. Diastolic blood pressure was measured at the fourth Korotkoff sound. Physical activity was assessed by a questionnaire (6,7).
Statistical analysis
Differences in physical and biochemical characteristics between Mexican and U.S. Pima Indians were tested by independent t test. Variables with skewed distribution were log transformed before analysis. We used multiple linear regressions to assess differences between groups in fasting insulin and HOMA-IR in separate models. Multivariate models were constructed by the stepwise method in its forward option using a P = 0.05. In addition, preliminary models were evaluated for possible interaction. The final models were tested for linear regression assumptions. In the pair-matched subset, differences in insulin resistance were analyzed by paired t test. All analyses were performed using STATA software (version 8.0; Stata Corp, College Station, TX) and P = 0.05 and P = 0.1 were used to test significance of selected covariates and their interaction terms.
Results
The study population consisted of 194 Mexican Pima Indians (94 men and 100 women) and 449 U.S. Pima Indians (203 men and 246 women) with normal glucose tolerance. As shown in Table 1, physical and biochemical characteristics were compared between Mexican and U.S. Pima Indians. Mexican Pima Indians were older and had higher physical activity (P < 0.0001) but had lower BMI, percent body fat, and waist circumference values than the U.S. Pima Indians (P < 0.0001). Fasting and 2-h glucose as well as 2-h insulin and HDL cholesterol levels were also significantly lower in the Mexican Pima Indians vs. U.S. Pimas (P < 0.0001). There were no differences in total cholesterol, triglycerides, and systolic and diastolic blood pressure (P > 0.05).
Table 1.
Physical and biochemical characteristics by population group with normal glucose tolerance
| Variable | Mexican Pima | U.S. Pima | P value |
|---|---|---|---|
| n | 194 | 449 | |
| Age (yr) | 36.8 ± 14.7 | 32.5 ± 10.0 | <0.0001 |
| BMI (kg/m2) | 24.6 ± 4.2 | 34.1 ± 8.0 | <0.0001 |
| Body fat (%) | 26.9 ± 11.0 | 39.6 ± 9.5 | <0.0001 |
| Waist circumference (cm) | 83.1 ± 11.0 | 107.5 ± 19.4 | <0.0001 |
| Fasting glucose (mg/dl) | 89.0 ± 8.8 | 92.9 ± 8.8 | <0.0001 |
| 2-h glucose (mg/dl) | 94.6 ± 21.4 | 104.0 ± 20.3 | <0.0001 |
| 2-h insulin (μU/ml)a | 21.8 (18.8, 25.4) | 64.1 (57.6, 71.4) | <0.0001 |
| HDL cholesterol (mg/dl) | 39.1 ± 9.4 | 44.7 ± 12.5b | <0.0001 |
| Total cholesterol (mg/dl) | 170.6 ± 37.1 | 173.0 ± 32.6 | 0.4234 |
| Triglycerides (mg/dl)a | 104.4 (96.8, 112.7) | 101.0 (93.4, 109.3)c | 0.5588 |
| SBP (mm Hg) | 115.7 ± 13.7 | 118.0 ± 15.3 | 0.0729 |
| DBP (mm Hg) | 71.3 ± 10.6 | 70.5 ± 11.1 | 0.3869 |
| PA (METs, h/wk)a | 131.7 (121.5, 142.8) | 32.7 (27.9, 38.8) | <0.0001 |
A t test was done for independent samples. To convert glucose to millimoles per liter, multiply by 0.0555; insulin to picomoles per liter, multiply by 6.945; HDL cholesterol to millimoles per liter, multiply by 0.0259; total cholesterol to millimoles per liter, multiply by 0.0259; triglycerides to millimoles per liter, multiply by 0.0113. SBP, Systolic blood pressure; DBP, diastolic blood pressure; PA, physical activity; MET, metabolic equivalent.
Geometric means (95% CI).
n = 168.
n = 143. All other data are presented as mean ± sd.
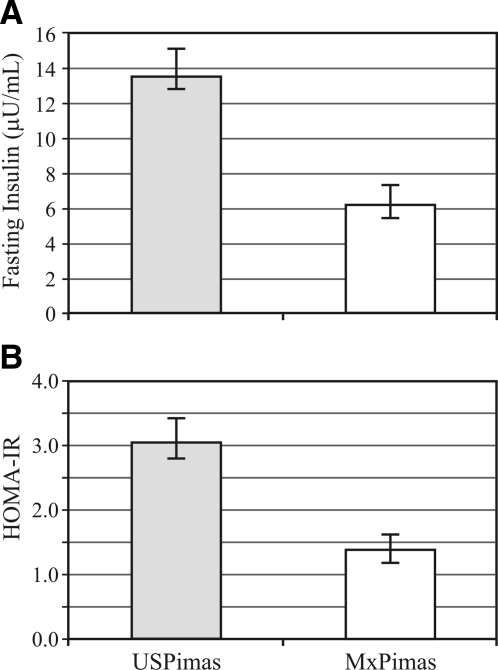
Results of the association between insulin resistance and group (U.S. Pima vs. Mexican Pima Indians) are presented in Fig. 1 as exponentiated means and 95% confidence interval (CI). The unadjusted analysis indicates that Mexican Pima Indians have lower mean fasting insulin (4.17, 95% CI 3.64–4.77 vs. 16.35, 95% CI 14.87–17.97 μU/ml) and HOMA-IR (0.91, 95% CI 0.79–1.05 vs. 3.73, 95% CI 3.38–4.11) than U.S. Pima Indians . Differences between groups remained significant either after adjustment for age, sex (fasting insulin: β = 1.339, 95% CI 1.172–1.506) and HOMA-IR (β = 1.387, 95% CI 1.216–1.559) or in a full model including BMI and waist circumference in addition to age and sex (fasting insulin: β = 0.779, 95% CI 0.579–0. 979) and HOMA-IR (β = 0.785, 95% CI 0.581–0. 989), taking the Mexican group as the reference. In the pair-matched subset (n = 79), large differences were confirmed [fasting insulin: 3.99, 95% CI 3.14–5.08 vs. 9.25, 95% CI 7.36–11.63 μU/ml and HOMA-IR: 0.89 (95% CI 0.70–1.14) vs. 2.02, (95% CI 1.60–2.56)]. Moreover, even though U.S. Pima Indians had similar age (31.18 vs. 30.99 yr), body fat (31.15 vs. 31.24%), and sex than Mexican Pimas, the groups differed considerably in physical activity expressed as metabolic equivalent hours per week (U.S. Pima: 46.16, 95% CI 32.22–66.14 vs. Mexican Pima: 133.93, 95% CI 113.70–152.40, P < 0.0001).
Figure 1.
Insulin resistance in U.S. Pima and Mexican Pima Indians with normal glucose tolerance measured by fasting insulin and HOMA-IR. Fasting insulin (A) and HOMA-IR (B) values are presented as exponentiated means with 95% CIs, adjusted for age, sex, BMI, and waist circumference. USPimas, U.S. Pima Indians; MxPimas, Mexican Pima Indians.
Discussion
Because U.S. Pima Indians have a marked increased prevalence of type 2 diabetes compared with their closely genetically related Mexican Pima group (6), we hypothesized that insulin resistance would be lower in the Mexican Pima Indians in comparison with that in the U.S. Pima Indians. Results of the present analysis showed that Mexican Pima Indians are less insulin resistant than their counterpart U.S. Pima Indians, even after adjusting for age, sex. and obesity. Furthermore, this finding was supported by the more than 2-fold difference in insulin resistance found using the pair-matched subset.
Part of the differences in insulin resistance between groups seems to be explained by the greater degree of obesity in the U.S. Pima Indians. The difference in insulin resistance between groups, although still significant, was reduced by about 40% after adjusting for obesity. With respect to fasting insulin, the adjusted predicted mean difference between group populations was 7.34 μU/ml (unadjusted mean difference 12.19 μU/ml), whereas that for HOMA-IR was 1.67 (unadjusted mean difference 2.82). Similarly, Dowse et al. (15) compared fasting insulin in different Asian populations and found that differences in insulin resistance between groups, although still significant, decreased considerably after controlling for total and central obesity as compared with that seen when controlling only for age. Furthermore and consistent with this observation, different studies have reported that obesity is one on the most important factors leading to insulin resistance (16).
Increasing age and female sex were also significantly associated with lower insulin resistance in the entire population. However, these two variables explained only a small amount of the difference in insulin resistance between both Pima groups. The adjusted coefficient value and the adjusted predicted mean difference between groups were attenuated by only 1.5% after adjusting for age and sex, a value much lower than the 40% difference after adjustment for obesity. Both age and sex, however, are nonmodifiable risk factors and inconsistent results have been found regarding their association with insulin resistance. In accord with our findings, an inverse association was shown between age and insulin concentration in both nondiabetic U.S. Pima Indians and Indians from Mauritius (17). However, no association between fasting insulin and age was found in a Native Canadian population (18).
Regarding the association between sex and insulin resistance, Hunt et al. (19) found lower fasting insulin concentrations in nondiabetic women in comparison with that in nondiabetic men but similar values in nondiabetic subjects with the metabolic syndrome. Conversely, Dowse et al. (15) showed that fasting insulin level was higher in women than that in men.
Physical activity was associated to insulin resistance in nondiabetic U.S. Pima Indians and Indians from Mauritius (17). Accordingly, the increased physical activity in Mexican Pima than in U.S. Pima Indians, even in those of similar age, body fat, and sex may also explain part of the differences in insulin resistance.
The main strength of this study is the fact that we are comparing two genetically related populations living with naturally contrasting lifestyles. Additional strengths are the facts that all the analysis followed similar measurement protocols in a central laboratory. Nevertheless, our study is limited by the cross-sectional nature of the data, which do not allow the demonstration any of temporal effect of the factors studied on risk of insulin resistance.
In conclusion, our results indicate that Mexican Pima Indians with normal glucose tolerance have lower insulin resistance in comparison with their genetically related counterparts U.S. Pima Indians, even after controlling for differences in obesity, age, and sex. This finding underscores the importance of lifestyle factors as direct protecting factors against insulin resistance in nondiabetic populations.
Acknowledgments
We thank the members of the Maycoba Pima Community and the Gila River Indian Community for their participation in these studies. Our gratitude goes to Bertha Isabel Pacheco, Ana Cristina Gallegos, and Hortensia Montesinos for data collection in Mexico and the staff of the Phoenix Epidemiology and Clinical Research Branch, National Institutes of Diabetes and Digestive and Kidney Diseases for data collection in Sacaton, AZ. Special thanks are extended to Nurse Eremita Perez Ruiz for facilitating work with our Mexican communities.
Footnotes
Disclosure Summary: The authors have nothing to disclose.
First Published Online July 28, 2010
Abbreviations: BMI, Body mass index; CI, confidence interval; HDL, high-density lipoprotein; HOMA-IR, homeostasis model assessment for insulin resistance.
References
- Martin BC, Warram JH, Krolewski AS, Bergman RN, Soeldner JS, Kahn CR 1992 Role of glucose and insulin resistance in development of type 2 diabetes: results of a 25-year follow-up study. Lancet 340:925–929 [DOI] [PubMed] [Google Scholar]
- Lillioja S, Mott DM, Spraul M, Ferraro R, Foley JE, Ravussin E, Knowler WC, Bennett PH, Bogardus C 1993 Insulin resistance and insulin secretory dysfunction as pre-cursors of non-insulin dependent diabetes mellitus: prospective studies of Pima Indians. N Engl J Med 329:1988–1992 [DOI] [PubMed] [Google Scholar]
- Weyer C, Hanson RL, Tataranni PA, Bogardus C, Pratley RE 2000 A high fasting plasma insulin concentration predicts type 2 diabetes independent of insulin resistance: evidence for a pathogenic role of relative hyperinsulinemia. Diabetes 49:2094–2091 [DOI] [PubMed] [Google Scholar]
- Knowler WC, Pettitt DJ, Saad MF, Bennett PH 1990 Diabetes mellitus in the Pima Indians: incidence, risk factors and pathogenesis. Diabetes Metab Rev 6:1–27 [DOI] [PubMed] [Google Scholar]
- Lillioja S, Nyomba BL, Saad MF, Ferraro R, Castillo C, Bennett PH, Bogardus C 1991 Exaggerated early insulin release and insulin resistance in a diabetes prone population: a metabolic comparison of Pima Indians and Caucasians. J Clin Endocrinol Metab 73:866–876 [DOI] [PubMed] [Google Scholar]
- Schulz LO, Bennett PH, Ravussin E, Kidd JR, Kidd KK, Esparza J, Valencia ME 2006 Effects of traditional and western environments on prevalence of type 2 diabetes in Pima Indians in Mexico and the USA. Diabetes Care 29:1866–1871 [DOI] [PubMed] [Google Scholar]
- Esparza J, Fox C, Harper IT, Bennett PH, Schulz LO, Valencia ME, Ravussin E 2000 Daily energy expenditure in Mexican and USA Pima Indians: low physical activity as a possible cause of obesity. Int J Obes Relat Metab Disord 24:55–59 [DOI] [PubMed] [Google Scholar]
- Tishkoff SA, Kidd KK 2004 Implications of biogeography of human populations for “race” and medicine. Nat Genet 36(Suppl 11):21–27 [DOI] [PubMed] [Google Scholar]
- World Health Organization Consultation Group 1999 Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1. Diagnosis and classification of diabetes mellitus. Geneva: World Health Organization; 59 [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC 1985 Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419 [DOI] [PubMed] [Google Scholar]
- Allain CC, Poon LS, Chan CS, Richmond W, Fu PC 1974 Enzymatic determination of total serum cholesterol. Clin Chem 20:470–475 [PubMed] [Google Scholar]
- Nägele U, Hägele EO, Sauer G, Wiedemann E, Lehmann P, Wahlefeld AW, Gruber W 1984 Reagent for the enzymatic determination of serum total triglycerides with improved lipolytic efficiency. J Clin Chem Clin Biochem 22:165–174 [DOI] [PubMed] [Google Scholar]
- Warnick GR, Benderson J, Albers JJ 1982 Dextran sulfate-magnesium precipitation procedure for quantitation of high-density lipoprotein cholesterol. Clin Chem 28:1379–1388 [PubMed] [Google Scholar]
- Rising R, Swinburn B, Larson K, Ravussin E 1991 Body composition in Pima Indians: validation of bioelectrical resistance. Am J Clin Nutr 53:594–598 [DOI] [PubMed] [Google Scholar]
- Dowse GK, Zimmet PZ, Alberti KG, Brigham L, Carlin JB, Tuomilehto J, Knight LT, Gareeboo H 1993 Serum insulin distribution and reproducibility of the relationship between 2-hour insulin and plasma glucose levels in Asian Indian, Creole, and Chinese Mauritians. Metabolism 42:1232–1241 [DOI] [PubMed] [Google Scholar]
- Goossens GH 2008 The role of adipose tissue dysfunction in the pathogenesis of obesity-related insulin resistance. Physiol Behav 94:206–218 [DOI] [PubMed] [Google Scholar]
- Kriska AM, Pereira MA, Hanson RL, de Courten MP, Zimmet PZ, Alberti KG, Chitson P, Bennett PH, Narayan KM, Knowler WC 2001 Association of physical activity and serum insulin concentrations in two populations at high risk for type 2 diabetes but differing by BMI. Diabetes Care 24:1175–1180 [DOI] [PubMed] [Google Scholar]
- Kriska AM, Hanley AJ, Harris SB, Zinman B 2001 Physical activity, physical fitness, and insulin and glucose concentrations in an isolated Native Canadian population experiencing rapid lifestyle change. Diabetes Care 24:1787–1792 [DOI] [PubMed] [Google Scholar]
- Hunt KJ, Williams K, Hazuda HP, Stern MP, Haffner SM 2007 The metabolic syndrome and the impact of diabetes on coronary heart disease mortality in women and men: the San Antonio Heart Study. Ann Epidemiol 17:870–877 [DOI] [PMC free article] [PubMed] [Google Scholar]