Abstract
In the title compound, C10H7ClN4, the quinoline ring system is planar [maximum deviation 0.0035 (10) Å]. The crystal structure is stabilized by van der Waals and π–π stacking interactions [centroid–centroid distance 3.6456 (17) Å].
Related literature
For quinoline derivatives as anti-tuberculosis agents, see: Jain et al. (2005 ▶).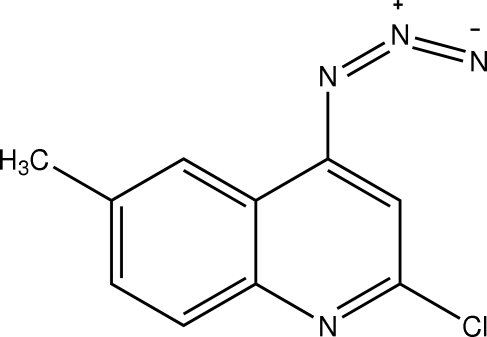
Experimental
Crystal data
C10H7ClN4
M r = 218.65
Triclinic,

a = 6.9517 (4) Å
b = 7.6078 (6) Å
c = 10.0191 (9) Å
α = 75.694 (7)°
β = 82.147 (8)°
γ = 76.532 (7)°
V = 497.57 (7) Å3
Z = 2
Mo Kα radiation
μ = 0.35 mm−1
T = 293 K
0.19 × 0.17 × 0.14 mm
Data collection
Nonius MACH-3 diffractometer
Absorption correction: ψ scan (North et al., 1968 ▶) T min = 0.935, T max = 0.952
2209 measured reflections
1743 independent reflections
1206 reflections with I > 2σ(I)
R int = 0.019
2 standard reflections frequency: 60 min intensity decay: none
Refinement
R[F 2 > 2σ(F 2)] = 0.057
wR(F 2) = 0.180
S = 1.08
1743 reflections
137 parameters
H-atom parameters constrained
Δρmax = 0.36 e Å−3
Δρmin = −0.35 e Å−3
Data collection: CAD-4 EXPRESS (Enraf–Nonius, 1994 ▶); cell refinement: CAD-4 EXPRESS; data reduction: XCAD4 (Harms & Wocadlo, 1996 ▶); program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: PLATON (Spek, 2009 ▶); software used to prepare material for publication: SHELXL97 .
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536809007041/at2729sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809007041/at2729Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Acknowledgments
SN thanks the DST for the FIST programme.
supplementary crystallographic information
Comment
Quinoline derivatives are a class of important materials as anti-tuberculosis agents (Jain et al., 2005). In the title molecule (Fig. 1), all non-H atoms of the molecule, except atoms Cl1, C10, N2, N3 and N4 are coplanar within 0.0035 (10) Å. Due to 4-azida substitution within the pyridine ring: C2═ C3 bond is longer and the C3—C4 bond is shorter than standard values for C═C (1.334 Å) and Csp2—Csp2 (1.455 Å) bond lengths respectively. The dihedral angle between the C3/N2-N4 and C2/C1/N1/C5 rings is 6.16 (11)°.
There is a weak π···π interaction observed between the centres of N1/C1—C5 rings related through the symmetry operator –x, 1-y, 1-z, with centroids separation of 3.6456 (17) Å.
Experimental
A mixture of 2,4-dichloroquinoline (2.12 g, 10 mmol) and sodium azide (0.650 g, 10 mmol) in DMF (20 ml) was refluxed for 2 h. The progress of the reaction was monitored by TLC. After conforming that the reaction got completed, the reaction mixture was cooled and poured on to the crushed ice with stirring. The solid settled was filtered to dryness and purified over a column of silica gel (60–120 mesh; 50 g) eluting with Petroleum Ether–ethyl acetate (4.5:1.5) to give 4-azido-2-chloro- 6-methylquinoline. The product was re-crystallized from 100% chloroform [mp: 429–430 K, yield: 20%].
Refinement
The H atoms were placed in calculated positions and allowed to ride on their carrier atoms with C—H = 0.93–0.96 Å and with Uiso = 1.2Ueq(C) for CH and Uiso = 1.5Ueq(C) for CH3 groups.
Figures
Fig. 1.
The molecular structure of the title compound, showing 50% probability displacement ellipsoids and the atom-numbering scheme.
Crystal data
| C10H7ClN4 | Z = 2 |
| Mr = 218.65 | F(000) = 224 |
| Triclinic, P1 | Dx = 1.459 Mg m−3 |
| Hall symbol: -P 1 | Mo Kα radiation, λ = 0.71073 Å |
| a = 6.9517 (4) Å | Cell parameters from 25 reflections |
| b = 7.6078 (6) Å | θ = 2–25° |
| c = 10.0191 (9) Å | µ = 0.35 mm−1 |
| α = 75.694 (7)° | T = 293 K |
| β = 82.147 (8)° | Block, colourless |
| γ = 76.532 (7)° | 0.19 × 0.17 × 0.14 mm |
| V = 497.57 (7) Å3 |
Data collection
| Nonius MACH-3 diffractometer | 1206 reflections with I > 2σ(I) |
| Radiation source: fine-focus sealed tube | Rint = 0.019 |
| graphite | θmax = 25.0°, θmin = 2.1° |
| ω–2θ scans | h = −1→8 |
| Absorption correction: ψ scan (North et al., 1968) | k = −8→9 |
| Tmin = 0.935, Tmax = 0.952 | l = −11→11 |
| 2209 measured reflections | 2 standard reflections every 60 min |
| 1743 independent reflections | intensity decay: none |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.057 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.180 | H-atom parameters constrained |
| S = 1.08 | w = 1/[σ2(Fo2) + (0.1143P)2 + 0.0878P] where P = (Fo2 + 2Fc2)/3 |
| 1743 reflections | (Δ/σ)max < 0.001 |
| 137 parameters | Δρmax = 0.36 e Å−3 |
| 0 restraints | Δρmin = −0.35 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| C1 | 0.1775 (5) | 0.7084 (4) | 0.2969 (3) | 0.0365 (8) | |
| C2 | 0.2459 (4) | 0.5180 (3) | 0.3035 (3) | 0.0334 (7) | |
| H2 | 0.2656 | 0.4702 | 0.2246 | 0.040* | |
| C3 | 0.2820 (4) | 0.4060 (3) | 0.4303 (3) | 0.0294 (7) | |
| C4 | 0.2518 (4) | 0.4825 (4) | 0.5495 (3) | 0.0291 (7) | |
| C5 | 0.1836 (4) | 0.6769 (4) | 0.5274 (3) | 0.0328 (7) | |
| C6 | 0.1508 (5) | 0.7579 (4) | 0.6436 (3) | 0.0432 (8) | |
| H6 | 0.1047 | 0.8853 | 0.6320 | 0.052* | |
| C7 | 0.1857 (5) | 0.6525 (4) | 0.7709 (3) | 0.0448 (9) | |
| H7 | 0.1636 | 0.7094 | 0.8454 | 0.054* | |
| C8 | 0.2548 (5) | 0.4583 (5) | 0.7950 (3) | 0.0407 (8) | |
| C9 | 0.2849 (4) | 0.3774 (4) | 0.6839 (3) | 0.0354 (7) | |
| H9 | 0.3283 | 0.2495 | 0.6978 | 0.043* | |
| C10 | 0.2945 (6) | 0.3461 (6) | 0.9386 (3) | 0.0581 (10) | |
| H10A | 0.1764 | 0.3662 | 0.9996 | 0.087* | |
| H10B | 0.3994 | 0.3836 | 0.9706 | 0.087* | |
| H10C | 0.3331 | 0.2169 | 0.9371 | 0.087* | |
| Cl1 | 0.13068 (16) | 0.85050 (11) | 0.13412 (9) | 0.0604 (4) | |
| N1 | 0.1475 (4) | 0.7888 (3) | 0.4003 (3) | 0.0395 (7) | |
| N2 | 0.3501 (4) | 0.2108 (3) | 0.4540 (3) | 0.0396 (7) | |
| N3 | 0.3912 (4) | 0.1499 (3) | 0.3461 (3) | 0.0418 (7) | |
| N4 | 0.4337 (5) | 0.0798 (4) | 0.2571 (3) | 0.0614 (10) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| C1 | 0.0458 (19) | 0.0243 (14) | 0.0406 (16) | −0.0080 (13) | −0.0078 (14) | −0.0063 (12) |
| C2 | 0.0435 (19) | 0.0236 (15) | 0.0374 (16) | −0.0062 (13) | −0.0066 (14) | −0.0138 (12) |
| C3 | 0.0317 (16) | 0.0202 (13) | 0.0404 (16) | −0.0029 (12) | −0.0044 (13) | −0.0160 (12) |
| C4 | 0.0304 (16) | 0.0214 (14) | 0.0388 (16) | −0.0021 (11) | −0.0035 (12) | −0.0158 (12) |
| C5 | 0.0359 (18) | 0.0227 (14) | 0.0432 (16) | −0.0032 (12) | −0.0034 (13) | −0.0165 (12) |
| C6 | 0.055 (2) | 0.0272 (16) | 0.053 (2) | −0.0039 (14) | −0.0025 (16) | −0.0249 (14) |
| C7 | 0.056 (2) | 0.0417 (18) | 0.0452 (19) | −0.0095 (16) | 0.0017 (16) | −0.0291 (15) |
| C8 | 0.044 (2) | 0.0449 (18) | 0.0385 (17) | −0.0112 (15) | −0.0041 (14) | −0.0171 (14) |
| C9 | 0.0400 (19) | 0.0255 (14) | 0.0426 (17) | −0.0018 (13) | −0.0064 (14) | −0.0139 (13) |
| C10 | 0.075 (3) | 0.062 (2) | 0.041 (2) | −0.013 (2) | −0.0078 (18) | −0.0161 (17) |
| Cl1 | 0.0950 (9) | 0.0351 (5) | 0.0482 (6) | −0.0092 (5) | −0.0201 (5) | −0.0002 (4) |
| N1 | 0.0535 (18) | 0.0188 (12) | 0.0460 (15) | −0.0010 (11) | −0.0058 (12) | −0.0121 (11) |
| N2 | 0.0572 (18) | 0.0208 (12) | 0.0413 (14) | 0.0034 (12) | −0.0084 (12) | −0.0164 (11) |
| N3 | 0.0582 (19) | 0.0204 (12) | 0.0487 (16) | −0.0013 (12) | −0.0104 (13) | −0.0146 (12) |
| N4 | 0.105 (3) | 0.0314 (14) | 0.0503 (17) | 0.0002 (16) | −0.0140 (17) | −0.0239 (13) |
Geometric parameters (Å, °)
| C1—N1 | 1.298 (4) | C6—H6 | 0.9300 |
| C1—C2 | 1.403 (4) | C7—C8 | 1.413 (4) |
| C1—Cl1 | 1.745 (3) | C7—H7 | 0.9300 |
| C2—C3 | 1.362 (4) | C8—C9 | 1.371 (4) |
| C2—H2 | 0.9300 | C8—C10 | 1.505 (5) |
| C3—N2 | 1.419 (3) | C9—H9 | 0.9300 |
| C3—C4 | 1.424 (3) | C10—H10A | 0.9600 |
| C4—C9 | 1.404 (4) | C10—H10B | 0.9600 |
| C4—C5 | 1.415 (4) | C10—H10C | 0.9600 |
| C5—N1 | 1.364 (4) | N2—N3 | 1.251 (3) |
| C5—C6 | 1.416 (4) | N3—N4 | 1.121 (3) |
| C6—C7 | 1.348 (4) | ||
| N1—C1—C2 | 126.1 (3) | C6—C7—C8 | 122.2 (3) |
| N1—C1—Cl1 | 117.0 (2) | C6—C7—H7 | 118.9 |
| C2—C1—Cl1 | 116.9 (2) | C8—C7—H7 | 118.9 |
| C3—C2—C1 | 117.2 (2) | C9—C8—C7 | 117.8 (3) |
| C3—C2—H2 | 121.4 | C9—C8—C10 | 121.7 (3) |
| C1—C2—H2 | 121.4 | C7—C8—C10 | 120.5 (3) |
| C2—C3—N2 | 124.0 (2) | C8—C9—C4 | 121.8 (3) |
| C2—C3—C4 | 120.3 (2) | C8—C9—H9 | 119.1 |
| N2—C3—C4 | 115.7 (2) | C4—C9—H9 | 119.1 |
| C9—C4—C5 | 119.6 (2) | C8—C10—H10A | 109.5 |
| C9—C4—C3 | 124.1 (2) | C8—C10—H10B | 109.5 |
| C5—C4—C3 | 116.3 (3) | H10A—C10—H10B | 109.5 |
| N1—C5—C4 | 123.2 (2) | C8—C10—H10C | 109.5 |
| N1—C5—C6 | 118.8 (2) | H10A—C10—H10C | 109.5 |
| C4—C5—C6 | 117.9 (3) | H10B—C10—H10C | 109.5 |
| C7—C6—C5 | 120.7 (3) | C1—N1—C5 | 116.7 (2) |
| C7—C6—H6 | 119.6 | N3—N2—C3 | 114.1 (2) |
| C5—C6—H6 | 119.6 | N4—N3—N2 | 173.6 (3) |
| N1—C1—C2—C3 | 0.9 (5) | C5—C6—C7—C8 | −0.4 (5) |
| Cl1—C1—C2—C3 | −179.7 (2) | C6—C7—C8—C9 | −0.5 (5) |
| C1—C2—C3—N2 | 179.5 (3) | C6—C7—C8—C10 | 179.2 (3) |
| C1—C2—C3—C4 | −0.4 (4) | C7—C8—C9—C4 | 1.0 (5) |
| C2—C3—C4—C9 | 179.8 (3) | C10—C8—C9—C4 | −178.6 (3) |
| N2—C3—C4—C9 | −0.1 (4) | C5—C4—C9—C8 | −0.7 (4) |
| C2—C3—C4—C5 | 0.0 (4) | C3—C4—C9—C8 | 179.5 (3) |
| N2—C3—C4—C5 | −179.9 (3) | C2—C1—N1—C5 | −0.9 (5) |
| C9—C4—C5—N1 | −179.9 (3) | Cl1—C1—N1—C5 | 179.7 (2) |
| C3—C4—C5—N1 | 0.0 (4) | C4—C5—N1—C1 | 0.4 (5) |
| C9—C4—C5—C6 | −0.1 (4) | C6—C5—N1—C1 | −179.3 (3) |
| C3—C4—C5—C6 | 179.7 (3) | C2—C3—N2—N3 | 5.8 (4) |
| N1—C5—C6—C7 | −179.6 (3) | C4—C3—N2—N3 | −174.3 (3) |
| C4—C5—C6—C7 | 0.7 (5) | C3—N2—N3—N4 | 176 (3) |
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: AT2729).
References
- Enraf–Nonius (1994). CAD-4 EXPRESS Enraf–Nonius, Delft, The Netherlands.
- Harms, K. & Wocadlo, S. (1996). XCAD4 University of Marburg, Germany.
- Jain, R., Singh, P. P., Jain, M., Sachdeva, S., Misra, V., Kaul, C. L., Kaur, S., Vaitilingam, B., Nayyar, A. & Bhaskar, P. P. (2005). Indian Patent Appl. IN 2002DE00628.
- North, A. C. T., Phillips, D. C. & Mathews, F. S. (1968). Acta Cryst. A24, 351–359.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Spek, A. L. (2009). Acta Cryst. D65, 148–155. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536809007041/at2729sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536809007041/at2729Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report



