Abstract
The role of membrane phosphatides in transport processes has been investigated in red cells from splenectomized patients with hereditary spherocytosis (HS).
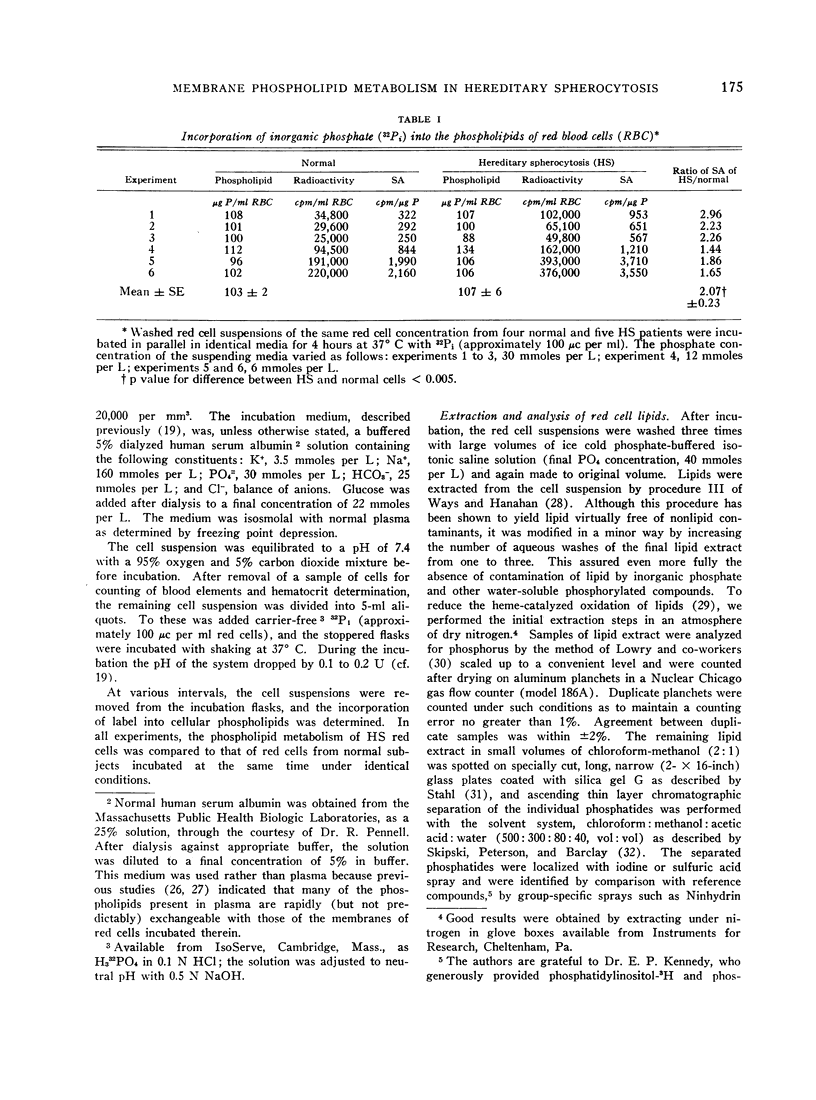
Incorporation of inorganic 32phosphate into the membrane phosphatides of HS red cells was approximately twice normal, coinciding with the nearly twofold increment in flux of sodium ions in the cells.
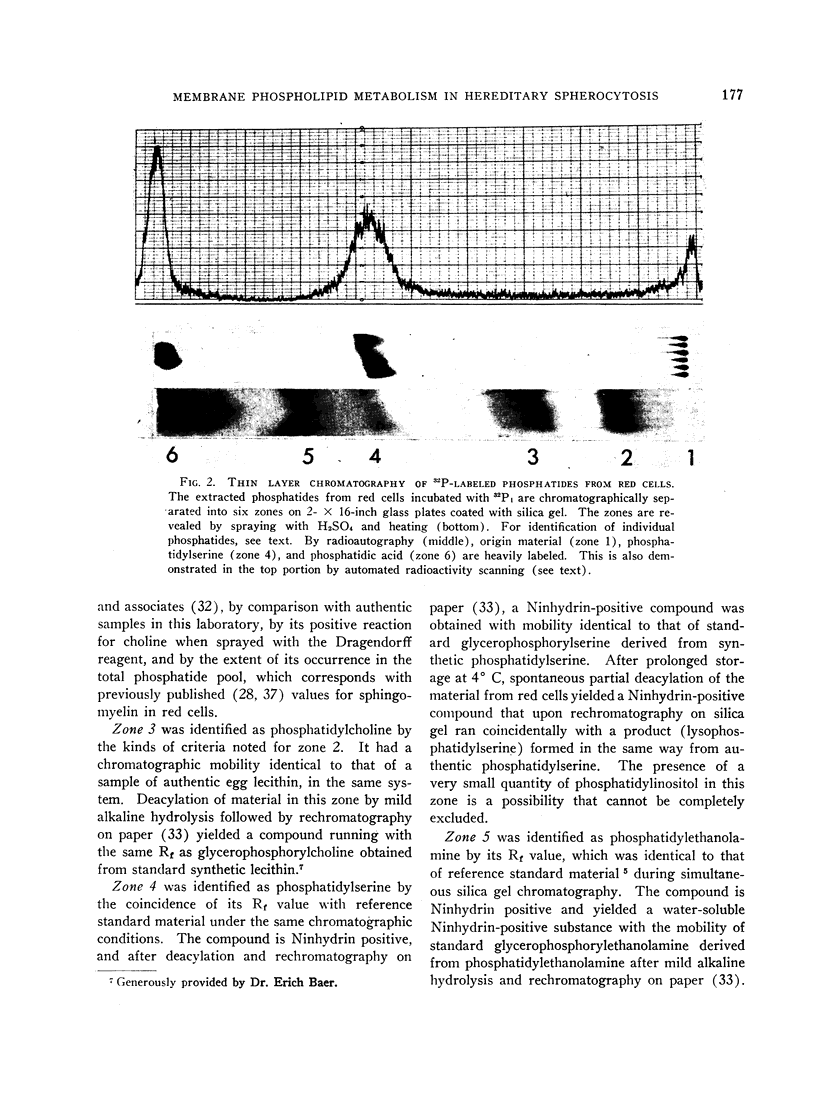
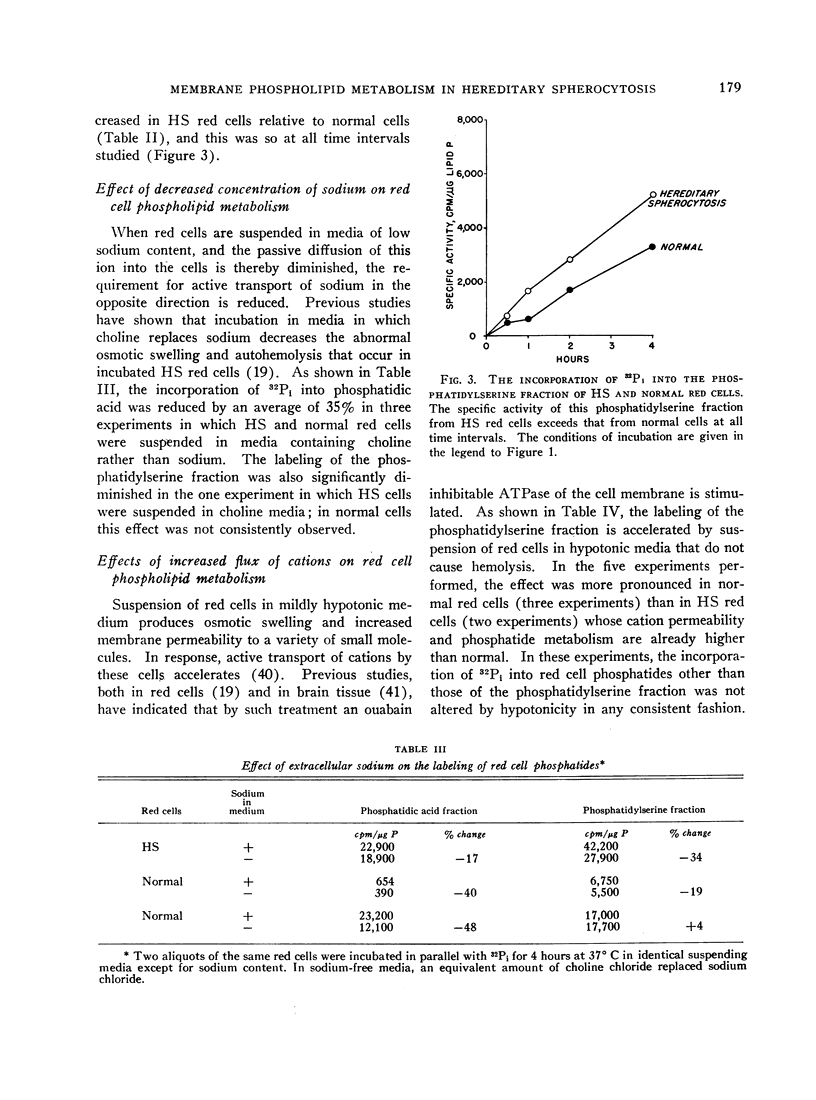
A consistent, inordinate increase in specific activity of a chromatographic fraction containing phosphatidylserine provided the bulk of the over-all increase in labeling of HS red cell phosphatides. The specific activity of phosphatidic acid was increased but not consistently.
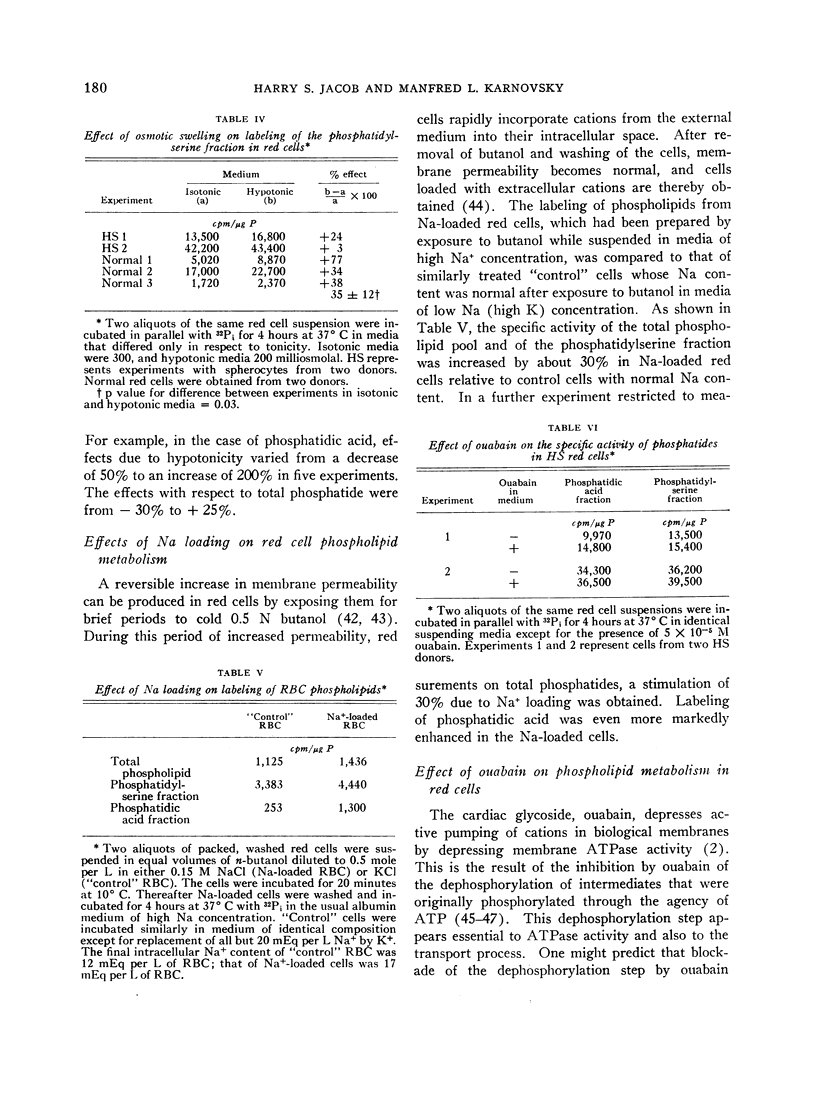
Radioactivity of the “acidic phosphatides” (phosphatidylserine and phosphatidic acid fractions) decreased, in general, when the sodium flux was low, i.e., when the cells were suspended in media of low sodium content. When the cation flux was elevated (hypotonic media), there was a marked (ca. 35%) increase in the labeling of phosphatidylserine fractions. Normal red cells whose permeability to cations was increased by exposure to 0.5 N butanol also exhibited increased labeling of acidic phosphatides.
Considerations of the stoichiometry of cation transport and phosphatide labeling make it unlikely that phospholipids act directly as carrier molecules for cations in red cell membranes. On the other hand, the involvement of these lipid substances in cation movements is substantiated by correlating several different states of sodium flux with the labeling of the phosphatidic acid and phosphatidylserine fractions.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAMSON M. B., KATZMAN R., WILSON C. E., GREGOR H. P. IONIC PROPERTIES OF AQUEOUS DISPERSIONS OF PHOSPHATIDIC ACID. J Biol Chem. 1964 Dec;239:4066–4072. [PubMed] [Google Scholar]
- AHMED K., JUDAH J. D. PREPARATION OF LIPOPROTEINS CONTAINING CATION-DEPENDENT ATPASE. Biochim Biophys Acta. 1964 Dec 9;93:603–613. doi: 10.1016/0304-4165(64)90343-5. [DOI] [PubMed] [Google Scholar]
- ALTMAN K. I., TABECHIAN H., YOUNG L. E. Some aspects of the metabolism of red blood cells from patients with hemolytic anemias. Ann N Y Acad Sci. 1958 Oct 13;75(1):142–147. doi: 10.1111/j.1749-6632.1958.tb36859.x. [DOI] [PubMed] [Google Scholar]
- Ahmed K., Judah J. D. Identification of active phosphoprotein in a cation-activated adenosine triphosphatase. Biochim Biophys Acta. 1965 Jun 15;104(1):112–120. doi: 10.1016/0304-4165(65)90227-8. [DOI] [PubMed] [Google Scholar]
- BERTLES J. F. Sodium transport across the surface membrane of red blood cells in hereditary spherocytosis. J Clin Invest. 1957 Jun;36(6 Pt 1):816–824. doi: 10.1172/JCI103487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenblum I., Chain E. An improved method for the colorimetric determination of phosphate. Biochem J. 1938 Feb;32(2):295–298. doi: 10.1042/bj0320295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CURRAN P. F., LIONETTI F., SOLOMON A. K. Possible cation-carrier substances in blood. Nature. 1956 Sep 15;178(4533):582–583. doi: 10.1038/178582a0. [DOI] [PubMed] [Google Scholar]
- DAWSON R. M., HEMINGTON N., DAVENPORT J. B. Improvements in the method of determining individual phospholipids in a complex mixture by successive chemical hydrolyses. Biochem J. 1962 Sep;84:497–501. doi: 10.1042/bj0840497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EMERSON C. P. The influence of the spleen on the osmotic behavior and the longevity of red cells in hereditary spherocytosis (congenital hemolytic jaundice); a case study. BMQ. 1954 Sep;5(3):65–76. [PubMed] [Google Scholar]
- FREINKEL N. Pathways of thyroidal phosphorus metabolism: the effect of pituitary thyrotropin upon the phospholipids of the sheep thyroid gland. Endocrinology. 1957 Oct;61(4):448–460. doi: 10.1210/endo-61-4-448. [DOI] [PubMed] [Google Scholar]
- GARDOS G. Akkumulation der Kaliumionen durch menschliche Blutkörperchen. Acta Physiol Acad Sci Hung. 1954;6(2-3):191–199. [PubMed] [Google Scholar]
- GARVIN J. E., KARNOVSKY M. L. The titration of some phosphatides and related compounds in a non-aqueous medium. J Biol Chem. 1956 Jul;221(1):211–222. [PubMed] [Google Scholar]
- GIBBS R., RODDY P. M., TITUS E. PREPARATION, ASSAY, AND PROPERTIES OF AN NA+- AND K+-REQUIRING ADENOSINE TRIPHOSPHATASE FROM BEEF BRAIN. J Biol Chem. 1965 May;240:2181–2187. [PubMed] [Google Scholar]
- GLYNN I. M., SLAYMAN C. W., EICHBERG J., DAWSON R. M. THE ADENOSINE-TRIPHOSPHATASE SYSTEM RESPONSIBLE FOR CATION TRANSPORT IN ELECTRIC ORGAN: EXCLUSION OF PHOSPHOLIPIDS AS INTERMEDIATES. Biochem J. 1965 Mar;94:692–699. doi: 10.1042/bj0940692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARRIS E. J., PRANKERD T. A. The rate of sodium extrusion from human erythrocytes. J Physiol. 1953 Sep;121(3):470–486. doi: 10.1113/jphysiol.1953.sp004959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOKIN L. E., HOKIN M. R. BIOLOGICAL TRANSPORT. Annu Rev Biochem. 1963;32:553–578. doi: 10.1146/annurev.bi.32.070163.003005. [DOI] [PubMed] [Google Scholar]
- HOKIN L. E., HOKIN M. R. Diglyceride kinase and other path ways for phosphatidic acid synthesis in the erythrocyte membrane. Biochim Biophys Acta. 1963 Mar 12;67:470–484. doi: 10.1016/0006-3002(63)91852-3. [DOI] [PubMed] [Google Scholar]
- HOKIN L. E., HOKIN M. R., MATHISON D. Phosphatidic acid phosphatase in the erythrocyte membrane. Biochim Biophys Acta. 1963 Mar 12;67:485–497. doi: 10.1016/0006-3002(63)91853-5. [DOI] [PubMed] [Google Scholar]
- HOKIN L. E., HOKIN M. R. Phosphatidic acid metabolism and active transport of sodium. Fed Proc. 1963 Jan-Feb;22:8–18. [PubMed] [Google Scholar]
- HOKIN L. E., SHERWIN A. L. Protein secretion and phosphate turnover in the phospholipids in salivary glands in vitro. J Physiol. 1957 Jan 23;135(1):18–29. doi: 10.1113/jphysiol.1957.sp005690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOB H. S., JANDL J. H. INCREASED CELL MEMBRANE PERMEABILITY IN THE PATHOGENESIS OF HEREDITARY SPHEROCYTOSIS. J Clin Invest. 1964 Aug;43:1704–1720. doi: 10.1172/JCI105046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JARNEFELT J. Some aspects of the physiological significance of the adenosinetriphosphatase of brain microsomes. Biochim Biophys Acta. 1962 Jun 4;59:655–662. doi: 10.1016/0006-3002(62)90645-5. [DOI] [PubMed] [Google Scholar]
- KARNOVSKY M. L., WALLACH D. F. The metabolic basis of phagocytosis. III. Incorporation of inorganic phosphate into various classes of phosphatides during phagocytosis. J Biol Chem. 1961 Jul;236:1895–1901. [PubMed] [Google Scholar]
- KENNEDY E. P. Biosynthesis of complex lipids. Fed Proc. 1961 Dec;20:934–940. [PubMed] [Google Scholar]
- KIRSCHNER L. B., BARKER J. TURNOVER OF PHOSPHATIDIC ACID AND SODIUM EXTRUSION FROM MAMMALIAN ERYTHROCYTES. J Gen Physiol. 1964 Jul;47:1061–1078. doi: 10.1085/jgp.47.6.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIRSCHNER L. B. The cation content of phospholipides from swine erythrocytes. J Gen Physiol. 1958 Nov 20;42(2):231–241. doi: 10.1085/jgp.42.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LARRABEE M. G., KLINGMAN J. D., LEICHT W. S. EFFECTS OF TEMPERATURE, CALCIUM AND ACTIVITY ON PHOSPHOLIPID METABOLISM IN A SYMPATHETIC GANGLION. J Neurochem. 1963 Aug;10:549–570. doi: 10.1111/j.1471-4159.1963.tb05053.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROBERTS N. R., LEINER K. Y., WU M. L., FARR A. L. The quantitative histochemistry of brain. I. Chemical methods. J Biol Chem. 1954 Mar;207(1):1–17. [PubMed] [Google Scholar]
- MOHLER D. N. ADENOSINE TRIPHOSPHATE METABOLISM IN HEREDITARY SPHEROCYTOSIS. J Clin Invest. 1965 Aug;44:1417–1424. doi: 10.1172/JCI105247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAGANO K., NAKAO M. Cation carrier in the erythrocyte membrane. J Biochem. 1962 Aug;52:99–102. doi: 10.1093/oxfordjournals.jbchem.a127588. [DOI] [PubMed] [Google Scholar]
- NICHOLLS D., KANFER J., TITUS E. The effect of ouabain on the incorporation of inorganic P32 into phospholipid. J Biol Chem. 1962 Apr;237:1043–1049. [PubMed] [Google Scholar]
- OHNISHI T., KAWAMURA H. R OLE DES PHOSPHATIDES DANS L'AD'ENOSINE TRIPHOSPHATASE SENSITIVE 'A L'OUABAINE LOCALIS'EE DANS LES MEMBRANES D'ERYTHROCYTE. J Biochem. 1964 Oct;56:377–378. doi: 10.1093/oxfordjournals.jbchem.a128006. [DOI] [PubMed] [Google Scholar]
- PARPART A. K., GREEN J. W. Potassium and sodium exchanges in rabbit red cells with n-butyl alcohol. J Cell Physiol. 1951 Dec;38(3):347–360. doi: 10.1002/jcp.1030380304. [DOI] [PubMed] [Google Scholar]
- PHILLIPS G. B., ROOME N. S. Quantitative chromatographic analysis of the phospholipids of abnormal human red blood cells. Proc Soc Exp Biol Med. 1962 Feb;109:360–364. doi: 10.3181/00379727-109-27203. [DOI] [PubMed] [Google Scholar]
- PRANKERD T. A. Studies on the pathogenesis of haemolysis in hereditary spherocytosis. Q J Med. 1960 Apr;29:199–208. [PubMed] [Google Scholar]
- REED C. F., SWISHER S. N., MARINETTI G. V., ENEN E. G. Studies of the lipids of the erythrocyte. I. Quantitative analysis of the lipids of normal human red blood cells. J Lab Clin Med. 1960 Aug;56:281–289. [PubMed] [Google Scholar]
- ROBINSON M. A., LODER P. B., DE GRUCHY G. C. Red-cell metabolism in non-spherocytic congenital haemolytic anaemia. Br J Haematol. 1961 Jul;7:327–339. doi: 10.1111/j.1365-2141.1961.tb00343.x. [DOI] [PubMed] [Google Scholar]
- ROWE C. E. The biosynthesis of phospholipids by human blood cells. Biochem J. 1959 Nov;73:438–442. doi: 10.1042/bj0730438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAKAGAMI T., MINARI O., ORII T. BEHAVIOR OF PLASMA LIPOPROTEINS DURING EXCHANGE OF PHOSPHOLIPIDS BETWEEN PLASMA AND ERYTHROCYTES. Biochim Biophys Acta. 1965 Feb 1;98:111–116. [PubMed] [Google Scholar]
- SAKAGAMI T., MINARI O., ORII T. INTERACTION OF INDIVIDUAL PHOSPHOLIPIDS BETWEEN RAT PLASMA AND ERYTHROCYTES IN VITRO. Biochim Biophys Acta. 1965 Apr 5;98:356–364. [PubMed] [Google Scholar]
- SEN A. K., POST R. L. STOICHIOMETRY AND LOCALIZATION OF ADENOSINE TRIPHOSPHATE-DEPENDENT SODIUM AND POTASSIUM TRANSPORT IN THE ERYTHROCYTE. J Biol Chem. 1964 Jan;239:345–352. [PubMed] [Google Scholar]
- SKOU J. C. ENZYMATIC BASIS FOR ACTIVE TRANSPORT OF NA+ AND K+ ACROSS CELL MEMBRANE. Physiol Rev. 1965 Jul;45:596–617. doi: 10.1152/physrev.1965.45.3.596. [DOI] [PubMed] [Google Scholar]
- Skipski V. P., Peterson R. F., Barclay M. Quantitative analysis of phospholipids by thin-layer chromatography. Biochem J. 1964 Feb;90(2):374–378. doi: 10.1042/bj0900374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosteson D. C., Cook P., Blount R. Separation of adenosine triphosphatase of HK and LK sheep red cell membranes by density gradient centrifugation. J Gen Physiol. 1965 Jul;48(6):1125–1143. doi: 10.1085/jgp.48.6.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WESTERMAN M. P., JENSEN W. N. IN VITRO INCORPORATION OF RADIOPHOSPHORUS INTO THE PHOSPHATIDES OF NORMAL HUMAN BLOOD CELLS. Proc Soc Exp Biol Med. 1965 Feb;118:315–319. doi: 10.3181/00379727-118-29830. [DOI] [PubMed] [Google Scholar]
- WHITTAM R. Potassium movements and ATP in human red cells. J Physiol. 1958 Mar 11;140(3):479–497. [PMC free article] [PubMed] [Google Scholar]
- WHITTAM R. The asymmetrical stimulation of a membrane adenosine triphosphatase in relation to active cation transport. Biochem J. 1962 Jul;84:110–118. doi: 10.1042/bj0840110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ways P., Hanahan D. J. Characterization and quantification of red cell lipids in normal man. J Lipid Res. 1964 Jul;5(3):318–328. [PubMed] [Google Scholar]
- YOSHIDA H., FUJISAWA H. Influence of subcellular structures on the activity of Na ion, K ion-activated adenosine triphosphatase in brain. Biochim Biophys Acta. 1962 Jul 2;60:443–444. doi: 10.1016/0006-3002(62)90431-6. [DOI] [PubMed] [Google Scholar]
- YOSHIDA H., NUKADA T., FUJISAWA H. Effect of ouabain on ion transport and metabolic turnover of phospholipid of brain slices. Biochim Biophys Acta. 1961 Apr 15;48:614–615. doi: 10.1016/0006-3002(61)90068-3. [DOI] [PubMed] [Google Scholar]
- de GIER, VAN DEENEN L., GEERDINK R. A., PUNT K., VERLOOP M. C. Phosphatide patterns of normal, spherocytic and elliptocytic red blood cells. Biochim Biophys Acta. 1961 Jun 24;50:383–384. doi: 10.1016/0006-3002(61)90347-x. [DOI] [PubMed] [Google Scholar]
- de Graeff J., Dempsey E. F., Lameyer L. D., Leaf A. Phospholipids and active sodium transport in toad bladder. Biochim Biophys Acta. 1965 Jul 7;106(1):155–170. doi: 10.1016/0005-2760(65)90104-9. [DOI] [PubMed] [Google Scholar]