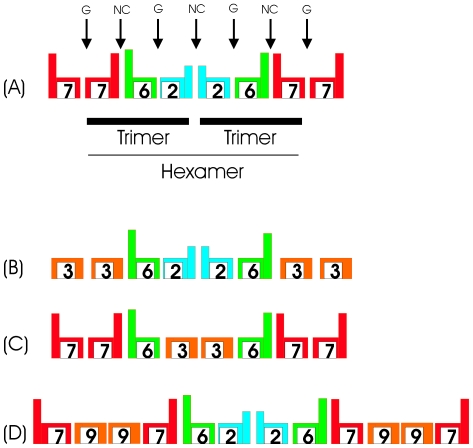
Figure 4. Schematic representation of possible septin-septin interactions within putative filaments, based on a combination of our yeast two hybrid assay's data and previously published data.
(A) the canonical or standard filament taken from the crystal structure of the 7-6-2 complex [18] showing the trimeric and hexameric cores and the NC and G interface. The 7-6 dimer is believed to be stabilized by a long coiled coil at an NC interface, whilst the 2-2 dimer is similarly stabilized by a short coiled coil. (B) possible arrangement for a filament composed of members from the SEPT3, SEPT6 and SEPT2 groups. SEPT3 group contains both septin 3 and 9. This arrangement would leave the SEPT6 coiled coil unpaired and “free” for interaction with other coiled-coil containing proteins (see text for details). (C) possible arrangement for a filament composed of members from the SEPT3, SEPT6 and SEPT7 groups. (D) possible position for SEPT9 compatible with the observed yeast two-hybrid data and data from the literature [38] (see also Fig. 3c,d).