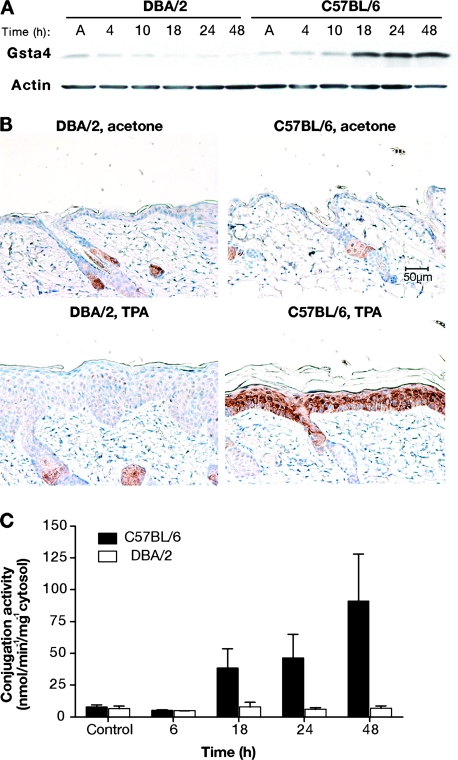
Figure 3.
Glutathione S-transferase α4 (Gsta4) protein expression and enzymatic activity in mouse skin following treatment with 12-O-tetradecanoylphorbol-13-acetate (TPA). A) Gsta4 protein levels in C57BL/6 vs DBA/2 epidermis following TPA treatment. Groups of three female C57BL/6 or DBA/2 mice were treated topically once with 6.8 nmol TPA or acetone. Mice were killed at the indicated time points and dorsal skin was removed. Epidermal cells were harvested by scraping over a chilled glass plate and then placed in radioimmunoprecipitation assay buffer before being homogenized using an 18-gauge needle and syringe. The homogenates were cleared by centrifugation and the supernatant was analyzed for protein content. Then, equal amounts of supernatant protein were subjected to western blot analysis of Gsta4 expression. Actin protein levels were also probed as a loading control. These results are representative of three independent studies. B) Immunohistochemical staining of Gsta4 in skin sections from DBA/2 and C57BL/6 mice. Mice were treated twice weekly for 2 weeks with either 6.8 nmol TPA or acetone and killed 24 hours after the final treatment. Dorsal skin was removed and samples were formalin fixed and embedded in paraffin before staining using hemotoxylin counterstain (blue) and a rabbit polyclonal anti-Gsta4 antibody and an horseradish peroxidase–conjugated anti-rabbit secondary antibody. The peroxidase reaction was assayed using diaminobenzidine as the chromagen (brown). Representative photomicrographs are presented (scale bar = 50 μm). C) 4-hydroxy-2(E)-nonenal (4-HNE) glutathione conjugation activity in epidermal GST preparations from TPA-treated C57BL/6 and DBA/2 mice. Groups of six C57BL/6 and DBA/2 mice were killed at the indicated time points following a single topical application of 6.8 nmol TPA, and the dorsal skins were removed. The epidermis was harvested and homogenized, and then cytosolic glutathione S-transferases were purified from the epidermal lysates using a glutathione–agarose column. Conjugation activity toward 4-HNE was assessed spectrophotometrically and normalized to protein content. The mean specific activity and 95% confidence interval are presented. These experiments were performed in duplicate with similar results.