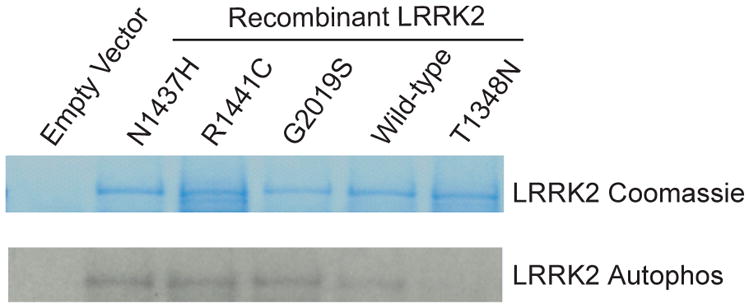
Figure 3. The p.Asn1437His mutation alters Lrrk2 GTP-binding and kinase activity.
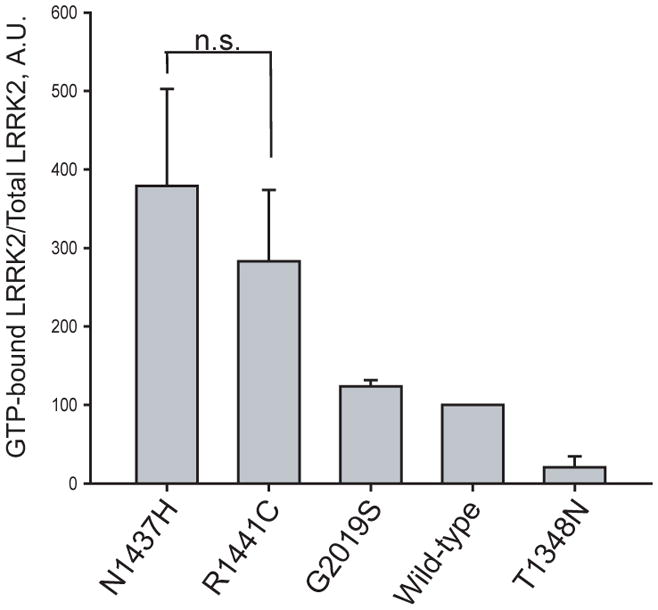
A) GTP-binding assay
GTP-binding assay, where the proportion of Lrrk2 bound to GTP-γ-sepharose beads normalized to total Lrrk2 in protein lysates shows that p.Asn1437His has a similar effect on activity compared with p.Arg1441Cys mutant Lrrk2 (n.s. is non-significant) and p.Asn1437His increases the proportion of GTP-bound Lrrk2 compared with wild-type protein. Data are derived from three independent experiments.
B) Lrrk2 kinase assay
Lrrk2 autophosphorylation kinase assay, with total Lrrk2 indicated by coomassie stain and kinase activity indicated by autoradiography. Normalized to input protein, p.Asn1437His demonstrates enhanced kinase activity compared with wild-type protein. The results are comparable to p.Gly2019Ser and p.Arg1441Cys Lrrk2 mutant proteins. Data are representative of three experiments.
