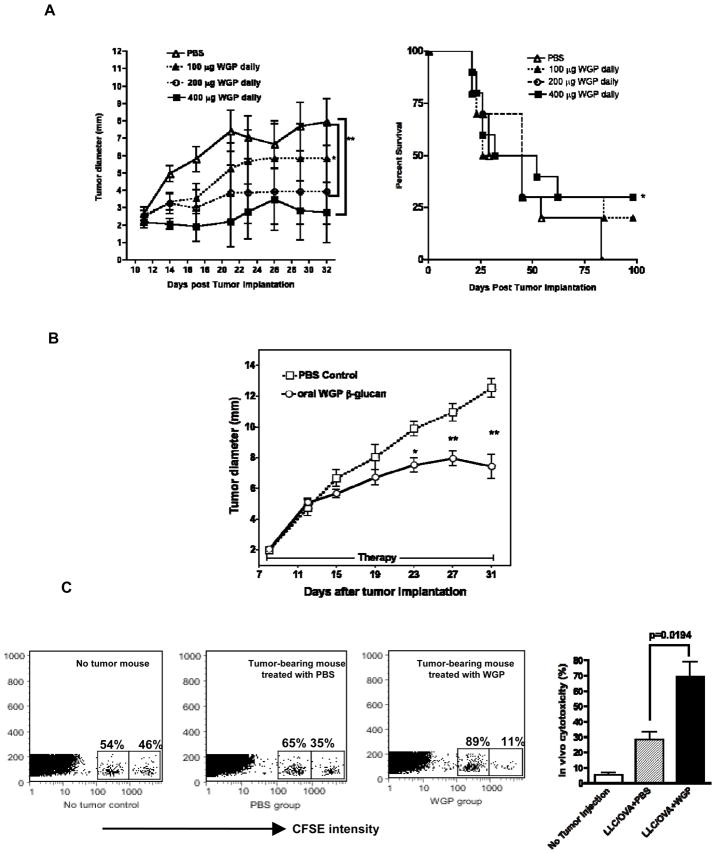
Figure 4. WGP treatment significantly reduces tumor burden and prolongs survival with increased CD8 T cell killing activity.
(A) Groups of mice (n=10) were implanted s.c. with RMA-S-MUC1, and after 11 days, to allow tumor formation, were treated with oral WGPs at different does (100 μg, 200 μg, 400 μg, daily) for 3 weeks. Tumor-free survival was also monitored. Points, mean; bars, SE. *p<0.05, **p<0.01. (B) Groups of mice (n=12) were implanted s.c with LLC/OVA tumor cells. After palpable tumors formed, mice were treated with or without orally administered WGPs for three weeks. Tumor diameters were recorded at the indicated time. *p<0.05, **p<0.01. Points, mean; bars, SE. (B) Tumor-bearing mice with LLC/OVA (n=3) treated with or without WGPs for three wks were adoptively transferred with OVA Class I peptide loaded CFSEhigh splenocytes with unloaded CFSElow splenocytes. Mice were sacrificed after 24 hrs and CFSE+ cells were gated and analyzed by flow cytometry. Data show that tumor-bearing mice treated with WGPs have the highest cytotoxicity against target cells compared to PBS treated or naïve mice.