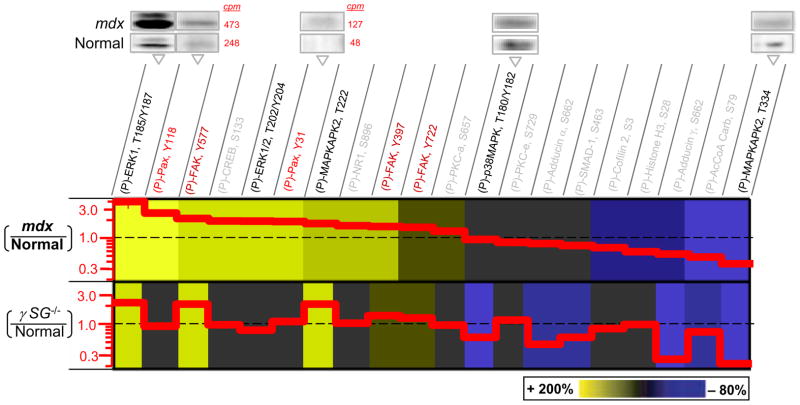
Figure 2.
Hyperphosphorylated MAPKs, FAK, and Paxillin in muscular dystrophies. Phosphoprotein immunoblots of normal (C57) and dystrophic (mdx) muscle tissue were quantified (with radio-labeled secondary) and normalized for direct comparison to results of γSG−/− (Griffin et al., 2005). Representative blot images are shown with cpm’s indicated for phosphopaxillins to illustrate the methods. Greater phosphorylation of paxillin is found in mdx. Increased levels of phospho-paxillin (Fluck et al., 1999; Melendez et al., 2004; Subauste et al., 2004), ERK (Most et al., 2003; Schaeffer et al., 2003; Mizukami et al., 2004; Palfi et al., 2005; Vittal et al., 2005; Das et al., 2006; Wei et al., 2006), and FAK (Lunn and Rozengurt, 2004; Lin et al., 2005; Peng et al., 2006) are associated with either survival or apoptosis, but only γSG−/− cells have been reported to be apoptotic (Griffin et al., 2005). Representative error bars for phospho-ERK are <20% (S.D./Avg.; n=3).