Table 2.
Anticonvulsant Activity and Toxicity of Compounds 8, 10, 13, 16, 17, 19–22, 24 Administered Orally (30 mg/kg) to Rats
| Compd | Structure | MESa | TOXb | ||||
|---|---|---|---|---|---|---|---|
| 0.25hc | 0.5hc | 1hc | 2hc | 4hc | |||
| 8 | 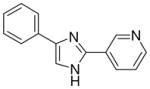 |
1/4 | 0/4 | 1/4 | 0/4 | 0/4 | 0/4 (−)d |
| 10 | 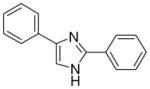 |
2/4 | 2/4 | 1/4 | 0/4 | 0/4 | 0/4 (−)d |
| 13 | 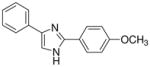 |
2/4 | 0/4 | 3/4 | 2/4 | 0/4 | 0/4 (−)d |
| 16 | 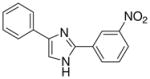 |
0/4 | 0/4 | 1/4 | 0/4 | 1/4 | 0/4(−)d |
| 17 | 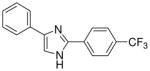 |
0/4 | 1/4 | 2/4 | 1/4 | 2/4 | 0/4(−)d |
| 19 | 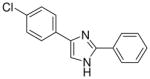 |
0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 (−)d |
| 20 | 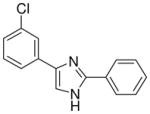 |
0/4 | 0/4 | 2/4 | 0/4 | 0/4 | 0/4 (−)d |
| 21 | 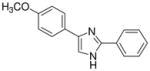 |
0/4 | 0/4 | 0/4 | 0/4 | 1/4 | 0/4 (−)d |
| 22 | 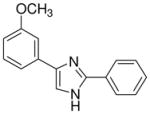 |
1/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 (−)d |
| 24 | 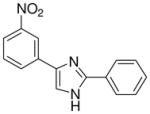 |
0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 (−)d |
Maximal electroshock test (number of animals protected/number of animals tested).
Neurotoxicity (number of animals protected/number of animals tested).
Time after drug administration.
(−) No neurotoxicity at doses tested.
