Abstract
Objective
The ability to imagine an elaborative event from a personal perspective relies on a number of cognitive processes that may potentially enhance subsequent memory for the event, including visual imagery, semantic elaboration, emotional processing, and self-referential processing. In an effort to find a novel strategy for enhancing memory in memory-impaired individuals with neurological damage, the present study investigated the mnemonic benefit of a method we refer to as “self-imagining” – or the imagining of an event from a realistic, personal perspective.
Method
Fourteen individuals with neurologically-based memory deficits and fourteen healthy control participants intentionally encoded neutral and emotional sentences under three instructions: structural-baseline processing, semantic processing, and self-imagining.
Results
Findings revealed a robust “self-imagination effect” as self-imagination enhanced recognition memory relative to deep semantic elaboration in both memory-impaired individuals, F (1, 13) = 32.11, p < .001, η2 = .71, and healthy controls, F (1, 13) = 5.57, p < .05, η2 = .30. In addition, results indicated that mnemonic benefits of self-imagination were not limited by severity of the memory disorder nor were they related to self-reported vividness of visual imagery, semantic processing, or emotional content of the materials.
Conclusions
The findings suggest that the self-imagination effect may depend on unique mnemonic mechanisms possibly related to self-referential processing, and that imagining an event from a personal perspective makes that event particularly memorable even for those individuals with severe memory deficits. Self-imagining may thus provide an effective rehabilitation strategy for individuals with memory impairment.
Keywords: Imagination, Self, Memory disorders, Memory rehabilitation, Episodic memory
Imagining an elaborative event from a personal perspective is a cognitive ability thought to involve numerous component processes. For example, if asked to imagine that you are at a college basketball game with a friend, you will presumably construct the spatial context of a basketball arena, place yourself somewhere in the context, and simulate watching the game with your friend. To do this, you will likely recruit cognitive processes such as visual (Rubin, Schrauf, & Greenberg, 2003) and spatial imagery (Hassabis, Kumaran, & Maguire, 2007) to construct the basketball arena, semantic and autobiographical memory retrieval to provide details about a college basketball game (Wheeler, Stuss, & Tulving, 1997), and self-referential processing to create experiential and emotional components associated with your personal viewpoint (Abraham, Schubotz, & von Cramon, 2008; Addis, Pan, Vu, Laiser, & Schacter, 2009; Szpunar, Watson, & McDermott, 2007). The use of these cognitive processes may facilitate the integration of multimodal information into an elaborative, personally-relevant, and vivid image, which may result in the event being very memorable. In an effort to discover a novel method for enhancing memory in individuals with neurological damage, the present study investigated the mnemonic utility of what we refer to as “self-imagining” – or the imagining of an elaborate event from a realistic, personal perspective.
Many of the cognitive processes presumably involved in self-imagining have been found to be successful methods for enhancing memory. For instance, visual imagery has long been known to be an effective encoding strategy for remembering a wide range of materials (Bower, 1970; for a review, see Glisky & Glisky, 2008) and has been used with numerous populations, including those with neurological damage (Manasse, Hux, & Snell, 2005; Thoene & Glisky, 1995; Twum & Parente, 1994). In most instances, visual imagery has been shown to be clearly superior to other encoding strategies such as rote rehearsal (Wilson, 1987; for a review, see Wilson & Kapur, 2008). One reason for the success of visual imagery as a mnemonic strategy is that it often involves the linking of a visual image to an appropriate retrieval cue such as a spatial location (i.e. method of loci), a keyword (i.e. peg-word method), or a distinguishing characteristic (i.e. for the learning of name-face associations). The readily accessible cues are then used later to retrieve the to-be-remembered material. Self-imagination may include the linking of the event to visual and spatial cues which may be subsequently used to trigger memory.
The mnemonic utility of self-imagination also may rely on benefits associated with semantic processing, because, when imagining, one may generate elaborations that expand on the semantic information incorporated in the episode. Numerous studies have demonstrated that processing information at a deep semantic level enhances memory relative to processing information at a shallow, perceptual level, and that further elaboration provides even greater benefits (Craik & Lockhart, 1972; Craik & Tulving, 1975). This phenomenon – known as the “levels of processing” (LOP) effect – has been demonstrated in memory impaired populations, including individuals with neurological damage although benefits are usually smaller in these groups (Cermak, 1982; Cermak & Reale, 1978; Keane et al., 1997). Self-imagination may result in the event being more semantically elaborated and meaningful and thus more memorable (Craik & Tulving, 1975).
It is further possible that the mnemonic utility of self-imagining may be influenced by an emotional component. It has been suggested that emotional responses are activated during the processing of information from a personal perspective (Northoff et al., 2006), and studies have shown repeatedly that emotion is capable of generating a mnemonic benefit (for a review, see Christianson, 1992; Davidson, McFarland, & Glisky, 2006; Kensinger, Garoff-Eaton, & Schacter, 2007; Maratos & Rugg, 2001), a phenomenon that may be referred to as an “emotional enhancement effect” (EEE). Furthermore, a number of studies have demonstrated that emotion can be used to improve memory in individuals with neurological damage (Burton, et al., 2004; Hamann, Cahill, McGaugh, & Squire, 1997; Hamann, Cahill, & Squire, 1997; Marquine, 2009; Phelps, LaBar, & Spencer, 1997). Self-imagination may intensify the emotional salience of the imagined event, and, as a result, emotional processing may play a role in the mnemonic utility of self-imagining.
Imagining an elaborative event from a realistic, personal perspective may depend on another cognitive process particularly effective at enhancing memory: self-referential processing. Indeed, one may need to reference one’s self knowledge in order to incorporate the thoughts, feelings, and sensory experiences that one might have when present at the imagined event. Numerous studies have demonstrated that processing information in reference to oneself is an exceptional strategy for enhancing memory (Bellezza, 1992; Klein, Loftus, & Burton, 1989; Maki & McCaul, 1985; Rogers, Kuiper, & Kirker, 1977). In fact, self-referential strategies have been found to enhance memory to a greater degree than other successful encoding strategies – a phenomenon often referred to as the “self-reference effect” (for a review, see Symons & Johnson, 1997) – and initial findings suggest that individuals with compromised memory functioning demonstrate benefits from self-referential processing as well. For instance, two recent studies have shown that self-referential encoding strategies successfully enhance memory in older adults (Glisky & Marquine, 2009; Gutchess, Kensinger, Yoon, & Schacter, 2007), a population that can be broadly characterized as experiencing some degree of memory decline. There is also preliminary evidence indicating that self-referential processing enhances memory in individuals with neurologically-based memory deficits. In an incidental encoding paradigm, Marquine and Glisky (2005) showed that a self-referential orienting task enhanced recognition memory for trait adjectives relative to semantic processing in individuals with neurological damage, and in a follow-up study Marquine (2009) replicated the memory improvement using a more difficult cued-recall task. We speculate that not only might the inclusion of self-relevant information enhance encoding, but the self might serve as a particularly effective retrieval cue. Furthermore, because knowledge of the self remains intact in at least some individuals with episodic memory impairments (Klein, Loftus, & Kihlstrom, 1996; Klein, Rozendal, & Cosmides, 2002; Marquine, 2009; Rathbone, Moulin, & Conway, 2009), self-imagining may be a particularly powerful memory strategy for memory-impaired individuals.
The benefits of self-referential encoding have also been investigated in combination with simple visual imagery in healthy, young adults, but findings have been mixed (Aron, Aron, Tudor, & Nelson, 1991; Brown, Keenan, & Potts, 1986; Czienskowski & Giljohann, 2002; Foley, Belch, Mann, & McLean, 1999; Lord, 1980; Lord, 1987). In an initial study, Lord (1980) instructed participants to imagine either the self interacting with an object from an outside of the body vantage point (i.e. observer or third person perspective) or a different person interacting with an object (i.e. Walter Cronkite or one’s father). Contrary to previous research using self-referential encoding strategies, in this study other-person imagery led to better memory than self-referential imagery. Although some follow-up studies have replicated Lord’s (1980) results (Aron et al., 1991; Czienskowski & Giljohann, 2002), others have demonstrated the more customary advantage for self-referential processing with imagery (Brown et al., 1986; Foley et al., 1999). The ambiguous results may be attributable in part to the fact that Lord (1980) and others instructed participants to envisage the self from an observer perspective, a manipulation that affects the phenomenology of imagery (Piolino et al., 2006; Robinson & Swanson, 1993) and likely reduces the realistic nature of the experience. Thus, whether the inclusion of self-referential processing in visual imagery enhances memory may depend on instructions, namely whether one imagines viewing oneself in the event (i.e., observer perspective) or imagines the event through one’s own eyes (i.e., field perspective). In addition, Foley et al. (1999) found that individuals spontaneously process other-person imagery in relation to the self. It may therefore be difficult to separate self-referential processing from other person imagery. 1
Although simple visual imagery has been shown to be effective in some neurological populations, other evidence suggests that imagining an elaborative episode may be impaired in individuals with neurological damage, particularly those with very severe memory deficits. Neuropsychology, patient studies, and neuroimaging findings converge on the conclusion that imagination involves regions of the brain that are implicated in the memory network (Addis, Wong, & Schacter, 2007; Botzung, Denkova, & Manning, 2008; for a review, see Schacter, Addis, & Buckner, 2008). Studies of amnesic patients provided an early indication that self-imagination might rely on the same brain regions as episodic memory. Tulving described the amnesic patient K.C. as incapable of remembering his past or imagining his future (Rosenbaum et al., 2005; Tulving 1985). Similar deficits in memory and imagination are mentioned in patients with Korsakoff’s amnesia (Talland, 1965), a patient with amnesia due to anoxic injury (Klein, Loftus, & Kihlstrom, 2002) and in a group of amnesic patients with isolated damage to the hippocampus (Hassabis, Kumaran, Vann, & Maguire, 2007). If memory-impaired individuals are deficient in self-imagination, then individuals with severe memory impairments may benefit less from the use of self-imagination as an encoding strategy in comparison to individuals with moderate to mild memory impairments. Alternatively, it may be the case that only individuals with relatively mild memory deficits will benefit at all from self-imagination. However, it remains an empirical question as to which individuals are most likely to benefit and under what conditions. For instance, previous research on imagination has demonstrated that the hippocampus plays a critical role in the ability to bind information into a spatially coherent image (Hassabis, Kumaran, Vann et al., 2007), whereas the left inferior frontal gyrus is involved in accessing relevant semantic information (Addis et al., 2009). Further, medial prefrontal cortex has been implicated in self-referential processing (Addis, Wong, & Schacter, 2007). Therefore the location of neurological damage may affect both the ability to construct spatially coherent and detailed images as well as the mnemonic benefits elicited by self-imagination.
The primary purpose of the present study was to investigate the potential utility of self-imagination as a mnemonic strategy for improving memory in individuals with neurological damage. The study was also designed to explore possible cognitive mechanisms of the self-imagination effect. We therefore included a levels of processing manipulation and used materials that portrayed either emotional or non-emotional events. In addition, because the self-imagination method had not been tested in normal individuals, we added a group of healthy individuals as a comparison group. We hypothesized that self-imagining would provide a greater mnemonic benefit than deep semantic processing. We also expected, consistent with previous literature, that there would be a LOP effect, which would be smaller in the patient group than in controls, and that emotional events would be remembered better than neutral events.
Method
Participants
Fourteen individuals, ages 34 to 67, with neurological damage of mixed etiology (10 TBI) and fourteen healthy controls, ages 30 to 67, participated in the study. The healthy control group did not differ from the individuals with neurological damage in age, education, or IQ (Table 2). Two additional individuals with neurological damage were enrolled in the study but withdrew prior to completion, and thus their data are not reported. Individuals were recruited from the pool of participants in our laboratory and from acquired brain injury support-groups in the community. Memory impairment was designated as a 1 standard deviation difference (i.e. 15 points) between pre-morbid intelligence measured with the North American Adult Reading Test (NAART) (Spreen & Strauss, 1998) and memory functioning measured with the general memory index (GMI) from the Wechsler Memory Scale III (WMS-III) (Wechsler, 1997). To be included in the study individuals with neurological damage had to have a memory impairment and be at least one year post-trauma. Table 1 shows the etiology, location of neurological damage, years that have passed since injury, gender, age, estimated pre-morbid IQ, GMI, and the size of the memory impairment (i.e. IQ-GMI) for each participant in the memory-impaired group. As can be seen, the majority of memory-impaired individuals (n = 8) have a severe memory disorder, with a GMI 30 or more points lower than their IQ. For memory-impaired participants 3, 4, 9, 13, and 14, information concerning lesion location was extracted from scan reports at time of injury, whereas participants 1, 2, 5, and 6 underwent scans within the past two years. All of the memory-impaired participants in the present study, however, are many years post-trauma, and the present status of their brains is uncertain. However, in the TBI cases, the damage is likely diffuse, including both frontal and temporal brain regions.
Table 2. Mean (SD) Descriptive Characteristics and Neuropsychological Data for Memory-Impaired and Healthy Control Participants.
| Memory-Impaired | Healthy Control | P-Value | |
|---|---|---|---|
| Descriptive Characteristics | |||
| Age | 47.5 (8.8) | 49.1 (11.8) | .69 |
| Education Level | 14.7 (2.6) | 15.0 (1.7) | .73 |
| Estimated IQ (NAART) | 108.8 (11.3) | 108.4 (4.7) | .90 |
| Executive Function Measures | |||
| Mental Control | 20.6 (7.7) | 27.9 (4.3) | <.01 |
| Mental Arithmetic | 9 (3.4) | 13.6 (2.3) | <.001 |
| Letter Fluency (FAS) | 30.6 (14.7) | 43.5 (11.1) | <.01 |
| Digit Span Backwards | 6.4 (3.2) | 8.4 (2.5) | .08 |
| Trails B (seconds) | 133.3 (62.4) | 53.9 (12.7) | <.001 |
| Modified WCST – Categories | 4.5 (2.14) | 5.5 (1.0) | .10 |
| Long Term Memory Measures | |||
| Logical Memory I – 1st Recall | 19.1 (9.1) | 32.6 (5.2) | <.001 |
| Faces I | 28.4 (8.2) | 40.1 (4.2) | <.001 |
| Verbal Paired Associates I | 12.9 (11.9) | 26.4 (4.6) | <.001 |
| CVLT – Long Delay Cued Recall | 6 (2.8) | 12.1 (2.8) | <.001 |
| Visual Paired Associates II | 4.4 (1.4) | 5.9 (0.5) | <.01 |
Notes. CVLT = California Verbal Learning Test; IQ = Intelligence Quotient; NAART = North American Adult Reading Test; WCST = Wisconsin Card Sorting Task
Table 1. Descriptive Characteristics for Memory-Impaired Participants.
| Participant | Etiology | Neurological Damage |
Years Since Injury |
Gender | Age | IQ | GMI | IQ – GMI |
|---|---|---|---|---|---|---|---|---|
| 1 | TBI | rFL/Diffuse | 26 | Female | 53 | 125 | 96 | 29 |
| 2 | TBI | rFL/rTL/Diffuse | 23 | Male | 44 | 125 | 110 | 15 |
| 3 | TBI | lTL/Diffuse | 21 | Female | 34 | 101 | 54 | 47 |
| 4 | Aneurysm | FLs | 21 | Male | 46 | 127 | 81 | 46 |
| 5 | TBI | FLs/rTL/Diffuse | 10 | Female | 47 | 118 | 98 | 20 |
| 6 | TBI | FLs/rTL/mPL | 18 | Male | 67 | 104 | 69 | 35 |
| 7 | TBI | 15 | Female | 53 | 103 | 63 | 40 | |
| 8 | TBI | 17 | Male | 42 | 107 | 70 | 37 | |
| 9 | TBI | FLs (r > l) | 26 | Female | 46 | 115 | 73 | 42 |
| 10 | TBI | 31 | Male | 50 | 97 | 78 | 19 | |
| 11 | TBI | 24 | Female | 38 | 98 | 51 | 47 | |
| 12 | Anoxia | 35 | Female | 54 | 98 | 79 | 19 | |
| 13 | Tumor | TLs/rFL | 11 | Female | 55 | 95 | 70 | 25 |
| 14 | Anoxia | TLs/FLs/Diffuse | 9 | Female | 36 | 110 | 70 | 40 |
|
| ||||||||
| Mean (SD) | 20.5(7.8) | 47.5(8.8) | 108.8(11.3) | 75.9(16.5) | 32.9(11.5) | |||
Notes. TBI = traumatic brain injury; r = right; l = left; m = medial; FL = frontal lobe; PL = parietal lobe; TL = temporal lobe
Neuropsychological Measures
In addition to the NAART, both memory-impaired and healthy control participants were administered a battery of neuropsychological tests which included measures of executive functioning and long term memory. The neuropsychological measures of executive functioning were the Modified Wisconsin Card Sorting Task (WCST) (Hart, Kwentus, Wade, & Taylor, 1988), Mental Control (WMS-III) (Wechsler, 1997), Mental Arithmetic from the Wechsler Adult Intelligence Scale – Revised (WAIS-R) (Wechsler, 1981), the FAS test of word fluency (Spreen & Benton, 1977), Digit Span Backwards (WMS-III) (Wechsler, 1997), and Trail Making Test Part B (Spreen & Strauss, 1998). Although the memory-impaired group received a full WMS-III and other memory tests, the control group received only a subset of those tests for comparison purposes. The neuropsychological measures of long term memory were Logical Memory I – First recall (WMS-III), Verbal Paired Associates I (WMS-III), Faces I (WMS-III), the California Verbal Learning Test (CVLT) Long Delay Cued Recall (Delis, Kramer, Kaplan, & Ober, 1987), and Visual Paired Associates II from the Wechsler Memory Scale – Revised (WMS-R) (Wechsler, 1987). Table 2 shows performance on the neuropsychological measures for the group of individuals with neurological damage and the healthy control participants. As expected, the group of individuals with neurological damage performed significantly worse relative to the control group on all measures of long term memory and most measures of executive functioning.
Materials
Experimental stimuli were 224 sentences that on average were 12 syllables in length (range = 7 to 19). Sentences were previously rated on concreteness (1 = very concrete to 9 = very abstract), pleasure (1 = very low to 9 = very high), and arousal (1 = very low to 9 = very high), and were divided into lists of 28 sentences matched on these variables (Davidson et al., 2006). The sentences were rated as highly concrete with a mean concreteness rating of 2.04. Sentences that received the highest scores on arousal and the lowest scores on pleasure were chosen as the emotional sentences. An example of an emotional sentence is “Ten thousand people died when the concert was bombed.” Sentences that received the lowest scores on arousal and intermediate scores on pleasure were chosen as neutral sentences. An example of a neutral sentence is “A street vendor was selling butter and other groceries.” Half of the sentences in each list were neutral and half were emotional with a negative valence. The neutral and emotional sentences were randomly mixed and were presented visually on a Dell laptop computer using DMDX (Forster & Forster, 2003).
Procedures
Participants provided written informed consent prior to taking part in the study. For the memory-impaired participants, the study was divided into two sessions, administered at least one week apart. Each session, approximately 45 minutes in length, required participants to intentionally encode sentences under two separate instructions. The first encoding condition was always a structural-baseline task, and the second encoding condition was either a semantic processing or self-imagining task. Each encoding condition began with a practice phase, consisting of 3 sentences, followed by the experimental task, which consisted of 28 target sentences (14 neutral, 14 emotional), presented between two primacy and two recency buffer sentences. The memory-impaired participants were presented with different sentences in each session and the sentences in each session were counter-balanced across participants such that each sentence appeared in each encoding condition and as an old or new sentence at test an equal number of times.
In the structural-baseline encoding condition, memory-impaired participants were instructed to count the number of syllables in the sentence and decide if there were more than 12 syllables. For each of the 28 sentences, the question “Does this sentence have more than 12 syllables?” appeared and the target sentence was placed below it. Participants had 10 seconds to record a decision for each trial and the sentence remained on the screen for the entire 10 seconds. This structural-baseline study phase took approximately 5 minutes to complete. After a two minute distracter task, which required counting backwards by 3s, participants took a self-paced yes-no recognition memory test for 56 sentences (28 new, 28 old).
In the semantic processing encoding condition, each of the 28 sentences was preceded by two context-setting sentences, and memory-impaired participants were instructed to “decide if the italicized [the target] sentence ‘fits in’ with the rest of the short story.” Participants were warned that only memory for the italicized sentence would be tested, but it was important to read the whole story before making a decision. A pilot study found that the participants could read on average 3 sentences within 10 seconds, and all participants reported that they were able to read the short stories within the allotted time. The two context sentences were similar in length to the italicized sentences and were chosen to match the emotionality of the target sentence. Half of the target neutral and emotional sentences were semantically congruent with the context sentences and therefore ‘fit in’ with the story, and half the target sentences were incongruent. An example of a semantically congruent trial is “She was writing a paper for her assignment. She did not know how to spell a word. She used a dictionary to help with her spelling.” An example of a semantically incongruent trial is “The man called the retail company. He ordered a new pair of shoes. She put the china in the cupboard.” Context sentences were counter-balanced across participants such that they were paired equally often with a group of congruent target sentences and another group of incongruent target sentences. The 5 minute semantic study phase was followed by 2 minutes of counting backwards and a self-paced yes-no recognition memory test for 56 sentences (28 new, 28 old).
In the self-imagining encoding condition, memory-impaired participants were instructed to “imagine you are at the scene described by the sentence. Imagine with as much detail as possible.” Pre-study practice trials ensured that the participants understood the instructions and how to engage in self-imagination. Participants were instructed to imagine each event as though they were physically at the scene of the event. They were encouraged to imagine the event from a personal perspective by including information such as sensory details, thoughts, and feelings that they themselves might have if they were present at the event. For each of the 28 sentences, the statement “Imagine this sentence” appeared and the target sentence was placed below it. Participants had 10 seconds to imagine the event, and the sentence remained on the screen for the entire 10 seconds. Participants were advised to close their eyes while imagining to aid image construction, but this was not required. A “beep” signaled that it was time to move on to the next trial. The self-imagining study phase took approximately 5 minutes to complete and was followed by two minutes of counting backwards and a self-paced yes-no recognition memory test for 56 sentences (28 new, 28 old).
After completion of the self-imagining session, memory-impaired participants were again presented the sentences from the self-imagining portion of the experiment. They were instructed to re-imagine the event and rate the image for vividness and detail using a 5-point scale ranging from “I cannot imagine the event at all” to “the event is very detailed and vivid.” The purpose of the imagery rating portion of the experiment was twofold: first, to ensure that all participants could consistently generate images, and second, to explore the possibility that imagery vividness was correlated with memory. The vividness rating portion of the experiment was administered after the initial study-test phase in order to control the presentation time during the study phase and keep it constant across conditions.
The experiment was modified for the control group, based on pilot testing, in order to make the difficulty of the memory task comparable to the task given to the memory-impaired individuals. All three tasks—structural-baseline, semantic processing, and self-imagining—were presented in a single session. The encoding conditions were blocked and administered in a continuous study phase that was approximately 20 minutes in duration. After a practice phase which consisted of 3 practice trials for each task, healthy control participants studied 28 sentences (14 neutral, 14 emotional) under each encoding condition, and each sentence was presented for 5 seconds. The structural-baseline task was always completed first, and the order of the semantic processing and self-imagining tasks was counterbalanced across participants. Two sentences were included at the beginning and end of the study phase to account for primacy and recency effects. Upon completing the study phase, participants were engaged in a 15 minute filled delay, which included a letter cancellation task, a digit span task, and a number cancellation task. After the delay, participants took a self-paced yes-no recognition memory test for 168 sentences (84 old, 84 new). Sentences were counterbalanced such that each sentence appeared in each encoding condition and as an old or new sentence an equal number of times. After the recognition memory test, participants were again presented the sentences from the self-imagining portion of the experiment and instructed to make vividness ratings using the same scale as the memory-impaired participants.
Results
Hit rates and false alarm rates for patients and controls are shown in Table 3. Because the healthy controls completed a single test phase, the mean false alarm rate for neutral materials (.12) was the same for all encoding conditions as was the mean false alarm rate for emotional materials (.15), and there was no difference between the two, t (13) < 1. For the memory-impaired group, there were different false alarm rates for each of the three conditions. A 3 (encoding condition) x 2 (emotion) repeated measures ANOVA of the false alarm rates revealed a significant effect of condition, F (2, 26) = 7.46, p < .05, η2 = .37, no effect of emotion, F (1, 13) < 1, and no interaction F (2, 26) = 1.27, p = .30. Subsequent contrasts indicated that false alarm rates in the self-imagining task were lower than false alarm rates in both the structural-baseline task, F (1, 13) = 9.26, p < .01, η2 = .42, and the semantic processing task, F (1, 13) = 10.43, p < .01, η2 = .45, and the latter two did not differ, F (1, 13) = 1.17, p = .30. Since false alarm rates were not equivalent across encoding conditions in the memory-impaired individuals, all further analyses were based on corrected recognition rates (i.e., hits minus false alarms). In addition, because the control group had only a single false alarm rate whereas the memory-impaired group had different false alarm rates across conditions, direct comparisons between the two groups could not be made. Thus, the corrected recognition data were analyzed separately for each group.
Table 3. Memory-Impaired Participants and Healthy Controls Mean (SD) Hit Rates and False Alarm Rates for Emotional and Neutral Sentences in the Structural, Semantic, and Self-Imagination Tasks.
| Patients |
Controls |
|||
|---|---|---|---|---|
| Encoding Task | Hits | False Alarms | Hits | False Alarms |
| Structural-Baseline | ||||
| Emotional Sentences | .57 (.23) | .19 (.18) | .60 (.27) | .15 (.14) |
| Neutral Sentences | .44 (.23) | .24 (.25) | .42 (.23) | .12 (.11) |
| Unelaborated Semantic | ||||
| Emotional Sentences | .66 (.21) | .22 (.23) | .79 (.22) | .15 (.14) |
| Neutral Sentences | .64 (.22) | .28 (.33) | .71 (.16) | .12 (.11) |
| Elaborated Semantic | ||||
| Emotional Sentences | .77 (.16) | .22 (.23) | .88 (.15) | .15 (.14) |
| Neutral Sentences | .82 (.19) | .28 (.33) | .88 (.12) | .12 (.11) |
| Self-Imagination | ||||
| Emotional Sentences | .92 (.12) | .12 (.17) | .97 (.05) | .15 (.14) |
| Neutral Sentences | .90 (.11) | .11 (.14) | .93 (.08) | .12 (.11) |
For the semantic encoding condition, sentences were divided into two groups according to whether they were rated as semantically congruent or semantically incongruent with their story contexts.2 Although both require semantic processing, congruent sentences can be more elaborated than incongruent sentences, and so we expected performance differences (Craik & Tulving, 1975; Staresina, Gray, & Davachi, 2009). We will refer to these as elaborated and unelaborated semantic processing conditions. Also, although the memory-impaired participants completed the structural-baseline task twice across the two sessions, performance did not differ as a function of session, t (13) < 1, and so results from the two structural-baseline tasks were collapsed. Corrected recognition data were analyzed for both groups using 4 (encoding task) x 2 (emotion) repeated measures ANOVAs.
Effects of Self-Imagining, Semantic Processing, and Emotion
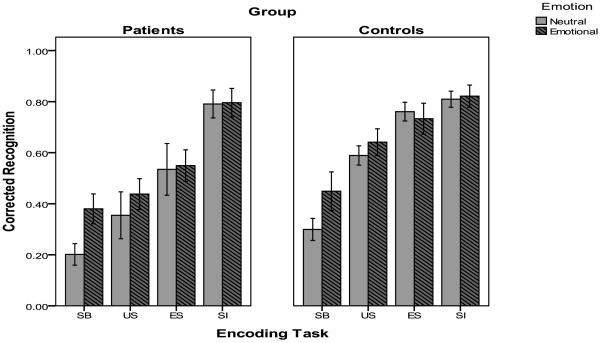
Corrected recognition results for all conditions and both groups are shown in Figure 1. For the memory-impaired individuals, there was a main effect of encoding task, F (3, 39) = 43.15, p < .001, η2 = .77. Subsequent contrasts indicated that self-imagining led to higher performance than elaborated semantic processing, F (1, 13) = 32.11, p < .001, η2 = .71, which in turn led to higher performance than unelaborated semantic processing, F (1, 13) = 17.72, p < .001, η2 = .58, which led to higher performance than in the structural-baseline condition, F (1, 13) = 4.90, p < .05, η2 = .27. There was a marginally significant main effect of emotion, F (1, 13) = 3.61, p = .08, η2 = .22 and no significant interaction between emotion and condition, F (3, 39) = 2.08, p = .12. Although the interaction did not reach significance, t-tests indicated that there was an emotional enhancement effect (EEE = .18) only in the structural-baseline condition, t (13) = 3.38, p < .01, r = .68.
Figure 1.
Corrected recognition for neutral and emotional materials in the structural-baseline (SB), unelaborated semantic (US), elaborated semantic (ES), and self-imagination (SI) conditions for patients and controls.
For healthy control participants, a repeated measures ANOVA also revealed a main effect of encoding task, F (3, 39) = 32.25, p < .001, η2 = .71. Similar to the memory-impaired individuals, subsequent contrasts indicated that self-imagining led to higher performance than elaborated semantic processing, F (1, 13) = 5.57, p < .05, η2 = .30, which in turn led to higher performance than unelaborated semantic processing, F (1, 13) = 10.25, p < .01, η2 = .44, which led to higher performance than in the structural-baseline condition, F (1, 13) = 22.08, p < .001, η2 = .63. There was no significant main effect of emotion, F (1, 13) = 1.79, p = .20, η2 = .12, nor was there a significant interaction, F (3, 39) = 2.04, p = .11, η2 = .16. However, the pattern of performance was similar to that of the memory-impaired group, suggesting that any emotion effect was restricted to the structural-baseline condition.
Relation between Memory Effects and Neuropsychological Function
We also performed exploratory analyses using Pearson product-moment correlations to examine the relation between each of the memory effects and performance on the neuropsychological measures. Because the mnemonic benefit of self-imagining was significantly greater than elaborated semantic processing, the SIE was defined as the mnemonic advantage of self-imagining relative to elaborated semantic processing. As such, the SIE was computed by subtracting the elaborated semantic processing condition from the self-imagining condition. The LOP effect was computed in the standard way by subtracting the structural-baseline condition from the semantic processing condition (collapsed across congruent and incongruent). In both cases, results were collapsed across neutral and emotional stimuli. Because the EEE was observed only in the structural baseline condition, the EEE was computed by subtracting performance on neutral sentences from performance on emotional sentences in the baseline condition only.
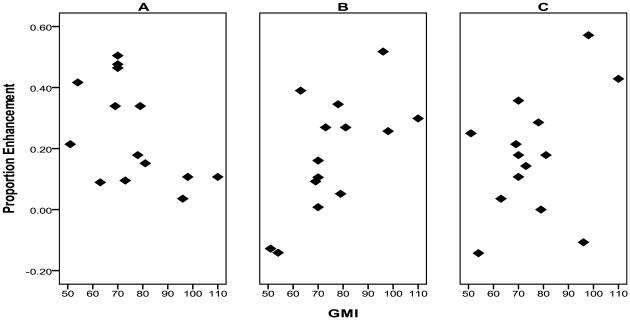
In the memory-impaired group, the size of the SIE was marginally correlated with the GMI from the WMS-III, r = −.50, p = .07. Those individuals with poorer memory functioning as measured by the GMI experienced a somewhat greater benefit from the self-imagining mnemonic strategy relative to elaborated semantic processing (see Figure 2a). The magnitude of the LOP effect was also related to memory functioning, r = .65, p = .01, but in this case, those individuals with better memory functioning as measured by the GMI benefited to a greater degree from deep processing relative to shallow processing (see Figure 2b). The size of the EEE was moderately related to the GMI, although not significantly, r = .41, p = .14. However, the trend in the data suggests that, as with LOP effects, those individuals with better memory functioning as measured by the GMI benefited to a greater degree from the emotionality of the sentence (see Figure 2c).
Figure 2.
Relation of memory functioning as measured by the general memory index (GMI) to a) the self-imagination effect (SIE), b) the levels of processing (LOP) effect, and c) the emotional enhancement effect (EEE).
Among the executive function tasks, the FAS test of verbal fluency was correlated marginally with the SIE, r = −.51, p = .08, and significantly with the LOP effect, r = .60, p < .05, and the Trail-Making Test Part B was correlated with LOP, r = −.66, p < .05. Better performance on these tasks was associated with greater LOP effects, while poorer performance (on FAS) was associated with a greater SIE. The EEE was not significantly correlated with any of the neuropsychological measures of executive functioning.
In control participants, the EEE was related to several neuropsychological tests of memory functioning, namely Faces I from the WMS – III, r = .57, p < .05, Verbal Paired Associates I from the WMS – III, r = .53, p = .05, and long delay cued recall from the CVLT, r = .47, p = .09. These findings suggest that, similar to the memory-impaired individuals, the control participants with better memory functioning experienced slightly greater benefits from the emotional content of the materials. Neither the SIE nor the LOP effect were significantly correlated with any of the neuropsychological measures of memory functioning or executive functioning in controls.
Imagery Effects
We examined whether the memory-impaired and healthy control individuals differed in their imagining ability. Imagery ratings ranged from 2 to 5 on a 5-point scale (1 = I cannot imagine the event at all to 5 = the event is very detailed and vivid) with a mean of 3.84 for the memory-impaired individuals and a mean of 3.86 for the healthy control individuals. The difference in mean imagery ratings between the groups was not significant, t (26) <1. We also examined the relation of imagery vividness and detail to the benefit of self-imagining. The size of the SIE was not significantly correlated with the rating of imagery detail and vividness for either the memory-impaired individuals, r = .19, p = .51, or the healthy control participants, r = −.24, p = .40.
Relation of the SIE to the EEE
To test the possibility that the mnemonic advantage generated by self-imagining might be related to emotional processing associated with imagining an event from a personal perspective, we compared the SIE for neutral materials (i.e., in the absence of emotion generated by the materials) to the EEE at baseline (i.e., in the absence of self-imagining or semantic processing). The size of the SIE for neutral materials was not related to the size of EEE at baseline for the memory-impaired individuals, r = −.25, p = .39.
Relation of the SIE to the LOP Effect
To investigate the possibility that the advantage of self-imagining might be related to benefits associated with deep semantic elaboration, we compared the SIE to the LOP effect. The SIE was significantly correlated with the magnitude of the LOP effect for the memory-impaired individuals, r = −.71, p < .01. Memory-impaired individuals who experienced minimal benefits from the semantic processing encoding strategy experienced greater benefits from the self-imagination mnemonic strategy. The magnitude of the SIE was not correlated with the LOP effect for the healthy control participants, r = −.29, p = .32.
Discussion
In an effort to find new ways to improve memory in individuals with neurological damage, the present study developed and tested a self-imagination encoding method that required participants to imagine to-be-remembered sentences from a realistic, personal perspective. Despite severe memory deficits, all 14 memory-impaired participants demonstrated a “self-imagination effect” or SIE. In addition, the present study found a similar self-imagination mnemonic advantage in a group of healthy individuals. These robust findings confirm our hypothesis that self-imagining would improve recognition memory relative to semantic processing.
To our knowledge, the present study is the first to demonstrate a memory enhancing effect for self-imagining in memory-impaired individuals with neurological damage. It appears that self-imagination of a scene makes the scene very memorable, so much so that even those individuals with very severe memory deficits benefited. In this study, participants were not constrained in the perspective they took while imagining. Nevertheless, participants reported that they usually viewed the scene through “their own eyes”—a field perspective—which has been found to be particularly effective for retrieval of detailed autobiographical memories (Bagri & Jones, 2009; Crawley & French, 2005; Piolino et al., 2006; Piolino et al., 2007). It appears that our instructions to include personal thoughts, feelings, and sensory experiences while imagining may have encouraged the use of a field perspective relative to an observer perspective. These findings are thus consistent with previous results showing that when information is processed from a field perspective and in relation to the self, it is particularly memorable.
The present study also attempted to investigate the mnemonic mechanisms associated with self-imagining. For instance, we tested whether the SIE may be partly attributable to emotional responses that are activated when information is processed from a personal perspective (Northoff et al., 2006). Numerous studies have shown that emotion enhances memory in individuals with neurological damage (Burton et al., 2004; Hamann, Cahill, McGaugh, & Squire, 1997; Hamann, Cahill, & Squire, 1997; Marquine, 2009; Phelps et al., 1997). Similarly, in the present study, we found enhanced memory performance for the memory-impaired individuals in the structural-baseline condition when the stimuli were emotional. However, in the self-imagining task, as well as in the semantic processing condition, non-emotional material was remembered as well as emotional material and there was no correlation between the EEE and the SIE. Similarly, in the healthy control participants there was a trend towards an EEE effect only in the structural baseline condition. We had speculated that emotional content might enhance memories for material encoded with self-imagination, because prior research had suggested that the benefit of processing information from a personal perspective might be related to an emotional component (Marquine, 2009; Northoff et al., 2006). However, in the present study, the correlation between the EEE and the SIE was small and non-significant (r = −.27 for controls and r = −.25 for patients), indicating that only a very small amount of the variance in the SIE was shared with the EEE. In addition, memory for emotional materials at baseline was well below the level of memory performance achieved with self-imagining for both the memory-impaired and healthy control individuals. Thus, although self-imagination may have an emotional component, it appears not to be the same component that is involved in the processing of emotional content. Consistent with the view that self-imagination from a personal perspective may involve a distinct emotional component, Eich and colleagues (2009) recently reported that increased activation of the amygdala was associated with a field perspective but not an observer perspective during retrieval of non-emotional autobiographical events. They suggested that a field perspective may thus involve an affective component that is independent of the valence of the material. Thus it is possible that the mnemonic benefits associated with our self-imagining condition are attributable to emotional responses that are associated particularly with the first-person perspective.
The failure to find an EEE for emotional sentences in the self-imagining condition might also be a consequence of a ceiling effect that might have masked the effect of emotion. However, recognition for sentences in the semantic processing conditions was well off the ceiling, and yet emotion did not enhance memory in these conditions either. It may be that the EEE associated with emotional stimuli is more likely to occur when other memory-enhancing encoding strategies such as semantic processing and self imagining are weak or absent.
In addition to an emotional component, the SIE may be partially credited to benefits associated with visual imagery. The self-imagining task involves creating a visual image, and as previously discussed, visual imagery is known to benefit memory. The data from the present study, however, are not completely consistent with previous findings regarding the mnemonic benefit of visual imagery in memory-impaired populations. First, in prior research the quality of visual imagery (i.e. concrete vs. abstract nouns) has been shown to mediate subsequent remembering in older adults, another population often experiencing memory problems (Dirkx & Craik, 1992). In contrast, the findings here indicate that the reported vividness of visual imagery was not related to the size of the SIE in the memory-impaired participants. This may reflect the questionable validity of subjective ratings, particularly those collected at the end of the test phase rather than during initial study (cf., Addis et al., 2009). On the other hand, other components of self-imagining may simply overwhelm the benefits of imagery alone. Second, individuals with the most severe memory deficits often benefit less from visual imagery encoding techniques (Gade, 1994; for a review, see Richardson, 1995; Wilson, 1987). Yet the findings from the present study suggest the opposite: the more severe the memory deficit, the greater the benefit from self-imagining. If the SIE was attributable only to visual imagery, then those individuals with the most severe memory deficits should have benefited less from self-imagining. Findings from the group of healthy control individuals provide additional evidence contradictory to an imagery explanation of the SIE. In fact, similar to the memory-impaired participants, imagery vividness was not correlated with the magnitude of the SIE in the healthy control participants. It thus does not appear that the advantages for self-imagination can be attributed solely to visual imagery.
Similarly, although self-imagination likely depends partly on a semantic processing component, the data cast doubt on the feasibility of a strict semantic elaboration explanation of the SIE. Despite the fact that a majority of the memory-impaired participants benefited mnemonically from the use of semantic processing at encoding, self-imagining had a significant mnemonic advantage over and above elaborated semantic processing, and the additional benefit was not correlated with the LOP effect in either the memory-impaired individuals or the healthy control participants. Thus self-imagining provided benefits well beyond typical LOP or semantic elaboration effects. 3 Furthermore, in contrast to the SIE, which provided the greatest benefits to those individuals with the most severe memory impairments, LOP provided greatest benefits for those individuals with the least severe memory deficits. The positive correlation between severity of memory impairment and the SIE may reflect the fact that those individuals with the most severe memory impairments experienced the smallest benefits from LOP and thus had more room to improve their memory in comparison to those individuals with less severe memory impairments. Nevertheless, unlike LOP, the SIE was not reduced by severity of memory impairment.
Although the mechanisms underlying the mnemonic benefits of self-imagination are not clear, one possibility is that they are related to the inclusion of self-referential processing in the imagination task. Since Rogers and colleagues (1977) first described the self-reference effect, a substantial amount of research has attempted to explain the mechanism behind the robust and reliable advantage of self-referential processing. Rogers et al. (1977) originally posited that the self possesses special mnemonic qualities, serving as a “superordinate schema” that permits access to unique encoding and retrieval mechanisms. More recently, Klein and colleagues (Klein, Cosmides, Costabile, & Mei, 2002) have provided evidence from neuropsychological patients that specialized learning systems are involved in the acquisition of information about one’s self. Functional neuroimaging research has shed further light on the issue, revealing that the neural correlates of self-referential processing are related to cortical midline structures, in particular the medial prefrontal cortex (mPFC) and precuneus (Amodio & Frith, 2006; Northoff et al., 2006; Szpunar et al., 2007), whereas semantic processing has usually been associated with left inferior prefrontal activations (Kapur et al., 1996; Poldrack et al., 1999; for a review, see Rugg, Otten & Henson, 2002). Although the debate continues as to whether the self is special (Gillihan & Farah, 2005; for earlier comments on this debate, see Brown et al., 1986, Greenwald & Banaji, 1989), these neuroimaging findings provide further evidence to support the notion that the self involves mechanisms other than those activated by semantic elaboration.
We suggest that involvement of the self in the imagining of events, particularly from a field perspective, taps special encoding and retrieval mechanisms, possibly located in cortical midline structures, which may be intact in our brain-injured patients. These special mechanisms may facilitate the synthesis of visual, semantic, and emotional information into a multimodal, personally-relevant memory for the imagined event, making it particularly memorable. Alternatively, they may strengthen links between to-be-remembered information and the self, the latter providing an especially effective retrieval cue. These ideas are of course speculative, and further research is needed to gain a better understanding of the mechanisms of self-imagining. For example, the present study cannot rule out the possibility that the mnemonic advantage of self-imagining is attributable to person processing in general and not the self per se (Gillihan & Farah, 2005). Nor can it evaluate whether a field perspective as compared to an observer perspective is critical for the SIE. Further experiments to explore these issues are now ongoing in our laboratory. In addition, location and extent of neurological damage was extracted from scan reports, and thus a more precise examination of what brain regions are intact in our group of memory-impaired individuals is not possible.
One question raised by the results of the present study is why almost all of the memory-impaired participants were able to consistently generate images while some past research suggests that imagining ability is deficient in memory-impaired persons. There are several possible explanations for this: First, there may be differences in the severity of the memory deficit across studies. Reports of individuals demonstrating a complete inability to imagine have come from studies of amnesic patients (Hassabis, Kumaran, Vann et al., 2007; Tulving, 1985), whereas our participants were characterized as memory-impaired. Nevertheless, based on a 30-point difference between IQ and GMI, 8 of the 14 memory-impaired patients in the present study could be classified as having severe impairments. Second, there may be differences in lesion location. Previous studies have almost all involved participants with damage confined to the hippocampus. In our sample, the majority of the memory-impaired participants had suffered traumatic brain injuries (TBIs), causing more diffuse damage, involving both temporal and frontal lobes. Third, in previous studies, people were asked either to imagine themselves in familiar places or to imagine their own personal future. These kinds of instructions may have induced greater retrieval from episodic/autobiographical memory than the instructions given in the present experiment, which may have induced retrieval of more semantic information. And fourth, the type of imagination instructions given to memory-impaired individuals may be very important. In the present study, participants were provided relatively detailed sentences to imagine, and they were not required to elaborate on external features of the episode such as the visual-spatial scenery. In fact, participants were instructed to imagine themselves at the scenes being described and to focus on personal or internal details, namely thoughts and feelings, relevant to the imagined episodes. Therefore, several aspects of the present study may account for why the memory-impaired participants appeared quite capable of self-imagining.
Another question posed by the findings of the present study is why self-imagination demonstrated a robust mnemonic enhancement when extensive evidence has indicated that autobiographical memory, a type of memory inherently related to the self, is typically impaired in individuals with neurological damage (Conway, 2005). One possibility is that self-imagination may rely on a different type of self-knowledge from autobiographical memory. In Conway’s cognitive model of self and memory (2005), autobiographical memory is purported to incorporate both an episodic self and a conceptual or semantic self. Numerous studies have shown that the mnemonic ability to “remember” personally experienced events – the episodic self – is often impaired in individuals with severe memory deficits. In contrast, several recent studies suggest that the ability to “know” facts about the self – the conceptual or semantic self – may be relatively intact in individuals with severe memory deficits. For example, Klein and colleagues (1996) describe patient W.J., a first-year college student who experienced a traumatic brain injury that caused a total loss of episodic autobiographical memory for the 7 months preceding the accident. Despite impairment in her ability to recall episodic autobiographical memories from her first year at college, W.J. accurately and reliably described herself and her personality during the same time period, an indication that conceptual knowledge of the self was intact. More recently, Rathbone, Moulin, and Conway (2009) presented similar findings from patient P.J.M., a 38 year old woman with retrograde amnesia caused by a traumatic brain injury. In this case study, P.J.M. was reportedly able to generate conceptual facts about the self, such as “I am a mum,” and “I am an academic,” but she very rarely could remember specific events related to these facts. Therefore, we propose that, although the ability to remember specific events from autobiographical memory may be impaired in brain-damaged individuals, these individuals may be able to draw upon conceptual or semantic self knowledge in order to simulate what one might think, feel, or personally experience at the scene of an imagined event. This processing of information in relation to the conceptual or semantic self along with the emotion that may be elicited from the personal perspective may elicit a mnemonic advantage.
Methodological differences between the present study and typical studies of autobiographical memory may also partially explain the discrepancy. The present study used a relatively short delay that spanned mere minutes, whereas in studies of autobiographical memory subjects are usually instructed to recall events that occurred across several lifetime periods, which may extend back decades. Thus, additional research is necessary to determine whether the benefits of self-imagining are sustainable over a longer delay. In addition, the discrepancy may be attributable to differences in memory task difficulty. While the present study used a recognition memory test, studies of autobiographical memory typically use cued-recall procedures, which may be more difficult. Thus, the advantage of self-imagining may be attenuated in a more difficult retrieval environment.
Finally, self-imagining benefited all memory-impaired individuals in the present study independent of etiology, lesion location, or memory function. Historically, mnemonic strategies have produced variable success across individuals with neurological damage (Glisky & Glisky, 2008), and the effectiveness of mnemonics is usually somewhat limited by the location and extent of damage (Wilson, 1987). The findings from the present study, although based on only 14 memory-impaired individuals, provide some optimism that this new method of self-imagination may have broader benefits among a wider range of neurological patients. However, the majority of memory-impaired individuals in the present study sustained their memory-impairments from traumatic brain injuries. Thus additional research is needed in order to demonstrate whether the benefits of self-imagining extend to memory-impaired individuals with different etiologies.
Conclusion
The findings from this study indicate that self-imagination can be a powerful method for enhancing memory, capable of providing a mnemonic advantage to individuals with even very severe memory deficits—an advantage comparable if not superior to that observed in those with much more moderate impairments. Based on these findings, there is reason to suspect that self-imagination may be a successful encoding strategy for people with a wide-range of memory impairments. Further studies are needed to replicate these results with larger numbers of memory-impaired participants of different etiologies and to extend the findings to memory tasks other than recognition. Nevertheless, the present study provides a promising beginning.
Our findings also provide further support for the notion that self-imagining recruits special encoding and retrieval mechanisms related to the self. The results from this study call into question the validity of a number of alternative explanations for the SIE, namely emotional processing, simple visual imagery, and deep semantic processing, thereby strengthening the view that self-imagination involves mechanisms more directly related to the self. Many successful rehabilitation methods such as vanishing cues (Glisky, 2004; Glisky, Schacter, & Tulving, 1986) and errorless learning (Baddeley & Wilson, 1994; Clare, Wilson, Carter, Roth, & Hodges, 2002) have capitalized on the fact that intact memory processes can compensate for damaged memory functions. If self-imagining taps into special encoding and retrieval mechanisms that are preserved in many individuals with neurologically-based memory deficits, then it may be possible to use those mechanisms to enhance memory when ordinary memory mechanisms are damaged. Although the precise nature of these mechanisms requires further investigation, something about imagining an event from one’s own perspective makes it an exceptional strategy for helping memory-impaired individuals remember.
Acknowledgments
This research was supported by National Institute on Aging Grant AG14792. We would like to thank Drs. John Allen and Lee Ryan for their feedback on an earlier version of this paper, and Dr. Maria Marquine for her help with recruitment and neuropsychological assessment. We also would like to thank the anonymous reviewers of this paper for their helpful comments and recommendations. This paper represents a portion of the Master’s thesis completed by M.D.G. in partial fulfillment of the requirements for a doctoral degree in the Psychology Department at the University of Arizona. Preliminary results from this paper were presented at the 2009 International Neuropsychological Society annual meeting.
Footnotes
Note that the ability to distinguish between imagining the self and imagining another person may require an observer perspective. Imagining an event through someone else’s eyes (i.e., from a field perspective), although conceptually possible, may involve theory of mind, which goes beyond simple imagining of another person, and is beyond the scope of the present paper.
Both patients and controls “misclassified” some sentences as congruent or incongruent, but there were no differences between groups. Patients misclassified 10.5% of sentences and controls misclassified 8.42% of sentences.
Note that if LOP and self-imagining are each compared to the same structural baseline, they are correlated significantly in memory-impaired individuals, r = .60, p < .05, and moderately though not significantly in healthy controls, r = .43, p = .12, suggesting that the overall benefit of self-imagining is partially attributable to semantic elaboration. The additional advantage of self-imagining, however, appears not to be related to the type of elaborative semantic processing that was required by our semantic processing task.
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/neu
References
- Abraham A, Schubotz RI, Yves von Cramon D. Thinking about the future versus the past in personal and non-personal contexts. Brain Research. 2008;1233:106–119. doi: 10.1016/j.brainres.2008.07.084. [DOI] [PubMed] [Google Scholar]
- Addis DR, Pan L, Vu M, Laiser N, Schacter DL. Constructive episodic simulation of the future and the past: Distinct subsystesms of a core brain network mediate imagining and remembering. Neuropsychologia. 2009 doi: 10.1016/j.neuropsychologia.2008.10.026. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Addis DR, Wong AT, Schacter DL. Remembering the past and imagining the future: common and distinct neural substrates during event construction and elaboration. Neuropsychologia. 2007;45(7):1363–1377. doi: 10.1016/j.neuropsychologia.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amodio DM, Frith CD. Meetings of minds: the medial frontal cortex and social cognition. Nature Reviews of Neuroscience. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Aron A, Aron E, Tudor M, Nelson G. Close relationships as including other in the self. Journal of Personality and Social Psychology. 1991;60:241–253. [Google Scholar]
- Baddeley A, Wilson BA. When implicit learning fails: amnesia and the problem of error elimination. Neuropsychologia. 1994;32(1):53–68. doi: 10.1016/0028-3932(94)90068-x. [DOI] [PubMed] [Google Scholar]
- Bagri G, Jones GV. Category-specific enhancement of retrieval due to field perspective. Memory. 2009;17(3):337–345. doi: 10.1080/09658210902740860. [DOI] [PubMed] [Google Scholar]
- Bellezza FS. Recall of congruent information in the self-reference task. Bulletin of the Psychonomic Society. 1992;30(4):275–278. [Google Scholar]
- Botzung A, Denkova E, Manning L. Experiencing past and future personal events: functional neuroimaging evidence on the neural bases of mental time travel. Brain and Cognition. 2008;66(2):202–212. doi: 10.1016/j.bandc.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Bower GH. Imagery as a relational organizer in associative learning. Journal of Memory and Language. 1970;9(5):529–533. [Google Scholar]
- Brown P, Keenan JM, Potts GR. The self-reference effect with imagery encoding. Journal of Personality and Social Psychology. 1986;51:897–906. [Google Scholar]
- Burton LA, Bernstein Vardy S, Frohlich J, Dimitri D, Wyatt G, Rabin L, Labar D. Affective tasks elicit material-specific memory effects in temporal lobectomy patients. Journal of Clinical and Experimental Neuropsychology. 2004;26(8):1021–1030. doi: 10.1080/13803390490515216. [DOI] [PubMed] [Google Scholar]
- Cermak LS. The long and short of it in amnesia. In: Cermak LS, editor. Human Memory and Amnesia. Erlbaum; Hillsdale, NJ: 1982. pp. 43–59. [Google Scholar]
- Cermak LS, Reale L. Depth of processing and retention of words by alcoholic Korsakoff patients. Journal of Experimental Psychology: Human Learning and Memory. 1978;4(2):165–174. [PubMed] [Google Scholar]
- Christianson SA. The handbook of emotion and memory. Erlbaum; Hillsdale, NJ: 1992. [Google Scholar]
- Clare L, Wilson BA, Carter G, Roth I, Hodges JR. Relearning face-name associations in early Alzheimer’s disease. Neuropsychology. 2002;16(4):538–547. doi: 10.1037//0894-4105.16.4.538. [DOI] [PubMed] [Google Scholar]
- Conway MA. Memory and the self. Journal of Memory and Language. 2005;53:594–628. [Google Scholar]
- Craik FIM, Lockhart RS. Levels of processing: A framework for memory research. Journal of Verbal Learning & Verbal Behavior. 1972;11:671–684. [Google Scholar]
- Craik FIM, Tulving E. Depth of processing and the retention of words in episodic memory. Journal of Experimental Psychology: General. 1975;104:268–294. [Google Scholar]
- Crawley SE, French CC. Field and observer viewpoint in remember-know memories of personal childhood events. Memory. 2005;13(7):673–681. doi: 10.1080/09658210444000296. [DOI] [PubMed] [Google Scholar]
- Czienskowski U, Giljohann S. Intimacy, concreteness, and the self-reference effect. Experimental Psychology. 2002;49:73–79. doi: 10.1027//1618-3169.49.1.73. [DOI] [PubMed] [Google Scholar]
- Davidson PS, McFarland CP, Glisky EL. Effects of emotion on item and source memory in young and older adults. Cognitive, affective & behavioral neuroscience. 2006;6:306–322. doi: 10.3758/cabn.6.4.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Kramer J, Kaplan E, Ober BA. The California Verbal Learning Test. Psychological Corporation; San Antonio, TX: 1987. [Google Scholar]
- Dirkx E, Craik FI. Age-related differences in memory as a function of imagery processing. Psychology and Aging. 1992;7(3):352–358. doi: 10.1037/0882-7974.7.3.352. [DOI] [PubMed] [Google Scholar]
- Eich E, Nelson AL, Leghari MA, Handy TC. Neural systems mediating field and observer memories. Neuropsychologia. 2009;47(11):2239–2251. doi: 10.1016/j.neuropsychologia.2009.02.019. [DOI] [PubMed] [Google Scholar]
- Foley MA, Belch C, Mann R, McLean M. Self-referencing: How incessant the stream? American Journal of Psychology. 1999;112:73–96. [Google Scholar]
- Forster KI, Forster JC. DMDX: a window display program with millisecond accuracy. Behavioral Research Methods, Instruments, & Computers: a Journal of Psychonomic Society, Inc. 2003;35(1):116–124. doi: 10.3758/bf03195503. [DOI] [PubMed] [Google Scholar]
- Gade A. Imagery as a mnemonic aid in amnesia patients: Effects of amnesia subtype and severity. In: Riddoch MJ, Humphreys GW, editors. Cognitive Neuropsychology and Cognitive Rehabilitation. Lawrence Erlbaum Associates, Inc.; Hillsdale, NJ, England: 1994. pp. 571–589. [Google Scholar]
- Gillihan SJ, Farah MJ. Is self special? A critical review of evidence from experimental psychology and cognitive neuroscience. Psychological Bulletin. 2005;131(1):76–97. doi: 10.1037/0033-2909.131.1.76. [DOI] [PubMed] [Google Scholar]
- Glisky EL. Disorders of memory. In: Ponsford J, editor. Cognitive and behavioral rehabilitation: From neurobiology to clinical practice. Guilford Press; New York, NY: 2004. pp. 100–128. [Google Scholar]
- Glisky EL, Glisky ML. Memory rehabilitation in older adults. In: Stuss DT, Winocur G, Robertson IH, editors. Cognitive Neurorehabilitation: Evidence and Application. Second Edition Cambridge Press; New York, NY: 2008. pp. 522–540. [Google Scholar]
- Glisky EL, Marquine MJ. Semantic and self-referential processing of positive and negative trait adjectives in older adults. Memory. 2009;17(2):144–157. doi: 10.1080/09658210802077405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glisky EL, Schacter DL, Tulving E. Learning and retention of computer-related vocabulary in memory-impaired patients: method of vanishing cues. Journal of Clinical and Experimental Psychology. 1986;8(3):292–312. doi: 10.1080/01688638608401320. [DOI] [PubMed] [Google Scholar]
- Greenwald AG, Banaji MR. The self as a memory system: Powerful, but ordinary. Journal of Personality and Social Psychology. 1989;57(1):41–54. [Google Scholar]
- Gutchess AH, Kensinger EA, Schacter DL. Functional neuroimaging of self-referential encoding with age. Neuropsychologia. 2009;48:211–219. doi: 10.1016/j.neuropsychologia.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutchess AH, Kensinger EA, Yoon C, Schacter DL. Ageing and the self-reference effect in memory. Memory. 2007;15(8):822–837. doi: 10.1080/09658210701701394. [DOI] [PubMed] [Google Scholar]
- Hamann SB, Cahill L, Squire LR. Emotional perception and memory in amnesia. Neuropsychology. 1997;2:104–113. doi: 10.1037//0894-4105.11.1.104. [DOI] [PubMed] [Google Scholar]
- Hamann SB, Cahill L, McGaugh JL, Squire LR. Intact enhancement of declarative memory for emotional material in amnesia. Learning and Memory. 1997;4:301–309. doi: 10.1101/lm.4.3.301. [DOI] [PubMed] [Google Scholar]
- Hart RP, Kwentus JA, Wade JB, Taylor JR. Modified Wisconsin Card Sorting Test in elderly normal, depressed, and demented patients. Clinical Neuropsychologist. 1988;2:49–56. [Google Scholar]
- Hassabis D, Kumaran D, Maguire EA. Using imagination to understand the neural basis of episodic memory. The Journal of Neuroscience. 2007a;27(52):14365–14374. doi: 10.1523/JNEUROSCI.4549-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassabis D, Kumaran D, Vann SD, Maguire EA. Patients with hippocampal amnesia cannot imagine new experiences. Proceeds of the National Academy of Science, USA. 2007b;104(5):1726–1731. doi: 10.1073/pnas.0610561104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur S, Tulving E, Cabeza R, McIntosh AR, Houles S, Craik FI. The neural correlates of intentional learning of verbal materials: a PET study in humans. Cognitive Brain Research. 1996;4(4):243–249. doi: 10.1016/s0926-6410(96)00058-4. [DOI] [PubMed] [Google Scholar]
- Keane MM, Gabrieli JDE, Monti LA, Fleischman DA, Cantor JM, Noland JS. Intact and impaired conceptual memory processes in amnesia. Neuropsychology. 1997;11(1):59–69. doi: 10.1037//0894-4105.11.1.59. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Garoff-Eaton RJ, Schacter DL. How negative emotion enhances the visual specificity of a memory. Journal of Cognitive Neuroscience. 2007;11:1872–1887. doi: 10.1162/jocn.2007.19.11.1872. [DOI] [PubMed] [Google Scholar]
- Klein SB, Cosmides L, Costabile KA, Mei L. Is there something special about the self? A neuropsycholigical case study. Journal of Research in Personality. 2002;36:490–506. [Google Scholar]
- Klein SB, Loftus J, Burton HA. Two self-reference effects: The importance of distinguishing between self-descriptiveness judgments and autobiographical retrieval in self-relevant encoding. Journal of Personality and Social Psychology. 1989;56(6):853–865. [Google Scholar]
- Klein SB, Loftus J, Kihlstrom JF. Memory and temporal experience: The effects of episodic memory loss on an amnesic patient’s ability to remember the past and imagine the future. Social Cognition. 2002;20:353–379. [Google Scholar]
- Klein SB, Loftus J, Kihlstrom JF. Self-knowledge of an amnesic patient: Toward a neuropsychology of personality and social psychology. Journal of Experimental Psychology: General. 1996;125(3):250–260. doi: 10.1037//0096-3445.125.3.250. [DOI] [PubMed] [Google Scholar]
- Klein SB, Rozendal K, Cosmides L. A social-cognitive neuroscience analysis of the self. Social Cognition. 2002;20(2):105–135. [Google Scholar]
- Lord CG. Schemas and images as memory aids: Two modes of processing social information. Journal of Personality and Social Psychology. 1980;38:257–269. [Google Scholar]
- Lord CG. Imagining self and others: Reply to Brown, Keenan, and Potts (1986) Journal of Personality and Social Psychology. 1987;38:445–450. [Google Scholar]
- Maki RH, McCaul KD. The effects of self-reference versus other reference on the recall of traits and nouns. Bulletin of the Psychometric Society. 1985;23:169–172. [Google Scholar]
- Manasse NJ, Hux K, Snell J. Teaching name-face associations to survivors of traumatic brain injury: A sequential treatment approach. Brain Injury. 2005;19:623–641. doi: 10.1080/02699050400013667. [DOI] [PubMed] [Google Scholar]
- Maratos EJ, Rugg MD. Electrophysiological correlates of the retrieval of emotional and non-emotional context. Journal of Cognitive Neuroscience. 2001;13:877–891. doi: 10.1162/089892901753165809. [DOI] [PubMed] [Google Scholar]
- Marquine MJ. Self-knowledge and self-referential processing in memory disorders: Implications for neuropsychological rehabilitation. Dissertation Abstracts International: Section B: The Sciences and Engineering. 2009;69:4432. [Google Scholar]
- Marquine MJ, Glisky EL. Self-knowledge and the self-reference effect in memory-impaired individuals; Paper presented at the International Neuropsychological Society; St. Louis, MO. 2005. [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain – A meta-analysis of imaging studies of the self. NeuroImage. 2006;31:440–457. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Phelps EA, LaBar KS, Spencer DD. Memory for emotional words following unilateral temporal lobectomy. Brain & Cognition. 1997;35:85–109. doi: 10.1006/brcg.1997.0929. [DOI] [PubMed] [Google Scholar]
- Piolino P, Desgranges B, Clarys D, Guillery-Girard B, Taconnat L, Isingrini M, Eustache F. Autobiographical memory, autonoetic consciousness, and self-perspective in aging. Psychology and Aging. 2006;21:510–525. doi: 10.1037/0882-7974.21.3.510. [DOI] [PubMed] [Google Scholar]
- Piolino P, Desgranges B, Manning L, North P, Jokic C, Eustache F. Autobiographical memory, the sense of recollection and executive functions after severe traumatic brain injury. Cortex. 2007;43(2):176–195. doi: 10.1016/s0010-9452(08)70474-x. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Wagner AD, Prull MW, Desmond JE, Glover GH, Gabrieli JDE. Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. NeuroImage. 1999;10:15–35. doi: 10.1006/nimg.1999.0441. [DOI] [PubMed] [Google Scholar]
- Rathbone CJ, Moulin CJA, Conway MA. Autobiographical memory and amnesia: Using conceptual knowledge to ground the self. Neurocase. 2009;15(5):405–418. doi: 10.1080/13554790902849164. [DOI] [PubMed] [Google Scholar]
- Richardson JTE. The efficacy of imagery mnemonics in memory remediation. Neuropsychologia. 1995;33(11):1345–1357. doi: 10.1016/0028-3932(95)00068-e. [DOI] [PubMed] [Google Scholar]
- Robinson JA, Swanson KL. Field and observer modes of remembering. Memory. 1993;1(3):169–184. doi: 10.1080/09658219308258230. [DOI] [PubMed] [Google Scholar]
- Rogers TB, Kuiper NA, Kirker WS. Self-reference and the encoding of personal information. Journal of Personality and Social Psychology. 1977;35(9):677–688. doi: 10.1037//0022-3514.35.9.677. [DOI] [PubMed] [Google Scholar]
- Rosenbaum RS, Kohler S, Schacter DL, Moscovitch M, Westmacott R, Black SE, Gao F, Tulving E. The case of K.C.: Contributions of a memory-impaired person to memory theory. Neuropsychologia. 2005;43:989–1021. doi: 10.1016/j.neuropsychologia.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Rubin DC, Schrauf RW, Greenberg DL. Belief and recollection of autobiographical memories. Memory & Cognition. 2003;31(6):887–901. doi: 10.3758/bf03196443. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Otten LJ, Henson RN. The neural basis of episodic memory: evidence from functional neuroimaging. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 2002;357(1424):1097–110. doi: 10.1098/rstb.2002.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Buckner RL. Episodic simulation of future events: Concepts, data, and applications. Annals of the New York Academy of Science. 2008;1124:39–60. doi: 10.1196/annals.1440.001. [DOI] [PubMed] [Google Scholar]
- Spreen O, Benton AL. Neurosensory Center Comprehensive Examination for Aphasia (NCCEA) University of Victoria Neuropsychology Laboratory; Victoria: 1977. [Google Scholar]
- Spreen O, Strauss E. A Compendium of Neuropsychological Tests. Second Edition Oxford University Press; New York, NY: 1998. [Google Scholar]
- Staresina BP, Gray JC, Davachi L. Event congruency enhances episodic memory encoding through semantic elaboration and relational binding. Cerebral Cortex. 2009;19:1198–1207. doi: 10.1093/cercor/bhn165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symons CS, Johnson BT. The self-reference effect in memory: A meta-analysis. Psychological Bulletin. 1997;121(3):371–394. doi: 10.1037/0033-2909.121.3.371. [DOI] [PubMed] [Google Scholar]
- Szpunar KK, Watson JM, McDermott KB. Neural substrates of envisioning the future. Proceeds of the National Academy of Science of the USA. 2007;104(2):642–647. doi: 10.1073/pnas.0610082104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talland GA. Deranged memory: A psychonomic study of the amnesic syndrome. Academic Press; New York and London: 1965. [Google Scholar]
- Thoene AIT, Glisky EL. Learning of name-face associations in memory-impaired patients: A comparison of different memory training procedures. Journal of the International Neuropsychological Society. 1995;1:29–38. doi: 10.1017/s1355617700000072. [DOI] [PubMed] [Google Scholar]
- Tulving E. Memory and consciousness. Canadian Journal of Psychology. 1985;26:1–12. [Google Scholar]
- Twum M, Parente R. Role of imagery and verbal labeling in the performance of paired associate tasks by persons with closed head injury. Journal of Clinical and Experimental Neuropsychology. 1994;16:630–639. doi: 10.1080/01688639408402674. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale-Revised. Psychological Corporation; New York: 1981. [Google Scholar]
- Wechsler D. The Wechsler Memory Scale-Revised. The Psychological Corporation; San Antonio, TX: 1987. [Google Scholar]
- Wechsler D. Wechsler Memory Scale-Third Edition: Administration and scoring manual. The Psychological Corporation; San Antonio, TX: 1997. [Google Scholar]
- Wheeler MA, Stuss DT, Tulving E. Toward a theory of episodic memory: The frontal lobes and autonoetic consciousness. Psychological Bulletin. 1997;121(3):331–354. doi: 10.1037/0033-2909.121.3.331. [DOI] [PubMed] [Google Scholar]
- Wilson BA. Rehabilitation of Memory. Guildford Press; New York, NY: 1987. [Google Scholar]
- Wilson BA, Kapur N. Memory rehabilitation for people with brain injury. In: Stuss DT, Winocur G, Robertson IH, editors. Cognitive Neurorehabilitation: Evidence and Application. Second Edition Cambridge Press; New York, NY: 2008. pp. 522–540. [Google Scholar]