Abstract
A central question in cancer biology is why most tumor susceptibility genes are linked with only limited types of cancer. Human germ-line mutation of the retinoblastoma susceptibility gene Rb1 is closely linked with just retinoblastoma and osteosarcoma, although the gene is universally expressed. Functional analysis of pRB and its close relatives, p107 and p130, has largely focused on their roles in repression of proliferation across all tissue types, but genetic evidence indicates an active requirement for pRB in osteoblast differentiation that correlates more directly with osteosarcoma susceptibility. Still, potential promoter targets of pRB and its role in normally differentiating osteoblasts remain insufficiently characterized. Here, an early marker of osteoblast differentiation, alkaline phosphatase, is identified as a direct promoter activation target of pRB. One role of pRB on this promoter is to displace the histone lysine demethylase KDM5A, thereby favoring trimethylation of H3K4, a promoter activation mark. A major new aspect of pRB-mediated transcriptional activation revealed in this promoter analysis is its role in recruitment of an activating SWI/SNF chromatin-remodeling complex. SWI/SNF is a critical coordinator of tissue-specific gene expression. In osteoblasts, SWI/SNF complexes containing the BRM ATPase repress osteoblast-specific genes to maintain the precursor state, while the alternative ATPase, BRG1, distinguishes an activating SWI/SNF complex necessary for RNA polymerase-II recruitment. A switch from BRM to BRG1 on the alkaline phosphatase promoter marks the onset of differentiation and is accomplished in a precise two-step mechanism. Dissociation of BRM-containing SWI/SNF depends on p300, and association of BRG1-containing SWI/SNF depends on pRB.
Keywords: pRB, SWI/SNF, Alpl, JARID1A, osteosarcoma, p300
INTRODUCTION
Human germ-line mutation of the retinoblastoma susceptibility gene, Rb1, is closely linked with only two types of cancer: retinoblastoma in early childhood and osteosarcoma in adolescence (1), even though the gene is universally expressed. The nature of the links between individual susceptibility genes and specific forms of cancer is a central question in cancer biology. Functional analysis of pRB and its close relatives, p107 and p130, has largely focused on their roles in repression of proliferation across all tissue types (2, 3), but genetic evidence indicates an active requirement for pRB in osteoblast differentiation that correlates more directly with osteosarcoma susceptibility (4, 5, 6, 7). Thomas et al. (5) demonstrated pRB in direct association with the activated promoters of the late-stage osteoblast markers osteocalcin and osteopontin, finding that pRB physically associates with and augments the activity of RUNX2 (alias CBFA1), a critical transcriptional regulator of osteoblast differentiation. More recently, pRB was shown to bridge interactions between RUNX2 and activating cofactors on the osteocalcin promoter (8, 9). pRB has also been shown to displace a target protein, KDM5A (aliases RBP2 and JARID1A), from the osteocalcin promoter to relieve repression and permit replacement by pRB and associated transactivators (10). Other osteoblast-specific promoter targets of pRB remain essentially uncharacterized, although earlier stages of differentiation represent the more likely point at which pRB deficiency leaves osteoblasts susceptible to tumorigenesis (1,6).
Induction of tissue-specific genes is critically dependent on the ATPase-powered SWI/SNF chromatin-remodeling complex, which repositions nucleosomes to block or unblock transcription (11). In mammals, an option of alternative subunits provides a means of distinguishing some SWI/SNF functions, with two choices for the ATPase core: BRM or BRG1. The essential role of BRG1 in activation of tissue-specific genes is well established, but we have recently shown that BRM-containing SWI/SNF exerts a counter-balancing repression function in osteoblast precursors, directly opposing the activating effect of BRG1 and helping to maintain the pre-osteoblast state until appropriate signals for terminal differentiation (12). pRB is thought to recruit SWI/SNF to repress cell cycle specific genes (13), but potential cooperation between pRB and SWI/SNF in gene activation has not been addressed.
These questions were approached using derivatives of the non-transformed pre-osteoblast cell line MC3T3-E1 (14, 15), engineered to express variants of the Adenovirus E1A oncogene. DNA tumor virus oncogenes such as E1A mimic pathways of carcinogenesis, making them highly informative physiological probes. The pRB family and the transcriptional co-activating acetyltransferases p300 and CBP are the critical targets of E1A functions that block differentiation and cell cycle arrest. They are targeted through genetically separable sites on E1A, so E1A variants can distinguish family-specific effects (Fig. 1A). These probes, in combination with chromatin immunoprecipitation analysis, identify the alkaline phosphatase gene, Alpl, as a direct promoter activation target of pRB. Alkaline phosphatase is the major early stage marker of osteoblast differentiation and is regulated coordinately with differentiation-associated cell cycle arrest. One role of pRB discernible on Alpl is to displace the repressor KDM5A/RBP2 , thereby favoring accumulation of a histone methylation signal associated with promoter activation. A major new aspect of pRB-mediated transcriptional activation revealed on Alpl is the essential role of pRB in recruitment of the activating BRG1-containing form of SWI/SNF.
Figure 1. Induction of alkaline phosphatase.
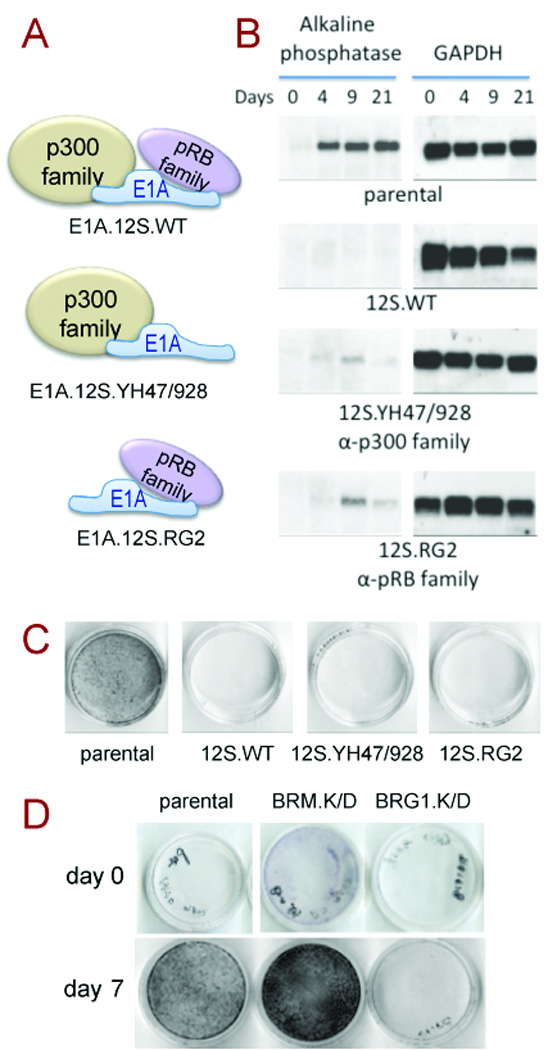
A. E1A 12S wild type (12S.WT) targets the p300 and pRB families. Mutant variants 12S.YH47/928 and 12S.RG2 carry missense mutations respectively inactivating binding of the pRB or p300 families.
B. Northern Blot showing the time course of alkaline phosphatase induction in normally differentiating osteoblasts.
C. In situ staining for alkaline phosphatase activity at day-7 post-induction.
D. In situ staining for alkaline phosphatase activity in lines where BRM or BRG1 are targeted by shRNA-mediated knockdown (K/D).
MATERIALS AND METHODS
Expression assays and cells
Low-passage MC3T3-E1 cells were a gift from Roland Baron (Yale University, New Haven, CT). Fresh aliquots thawed from liquid nitrogen are generally passaged for no more than three months. Each new aliquot is authenticated for the osteoblast phenotype by staining for alkaline phosphatase and the mineralization phenotype following induction with ascorbic acid and β- glycerol phosphate (4). Northern blotting, in situ staining for alkaline phosphatase activity, differentiation of MC3T3-E1 cells by exposure to ascorbic acid and β-glycerol phosphate, and generation of the BRM.GG5 and BRG1.D16 knockdown lines have been described (4, 12, 15). Lines expressing E1A 12S.RG2 were isolated as described for 12S.YH47/928 (4). pRB-depleted lines were generated using the Origene HuSH system and puromycin selection. Sequences 1 and 2 respectively are: CCTGTGCTCTTGAAGTTGTAATGGCTACG and CCTTACGGATTCCTGGAGGTAACATCTAT.
ChIP assay
Primers 5’- GGCTGGGACAGACAGAATGT and 5’- CTGCAACAGGCAGGGTAAC amplify the Alpl sequence between −163 and −535 (16). Primers 5’TTG TTC CTC TTG CCT CAG GT and 5’-TGA CAA TCA CAT GGC CTC TC amplify a sequence in the 3’UTR of Alpl (17). Antibodies were obtained either from Santa Cruz: pRB (sc-50x), p107 (sc- 318), p300 (sc-584), CBP (sc-583), BRM (sc-6450), BRG1 (sc-10768), TFIIB (sc-225), TFIID (sc-204), and RNA polymerase-II (sc-9001), Millipore: monomethyl-histone H3 lysine 4 (07-436), dimethylhistone H3 lysine 4 (07-030), trimethyl-histone H3 lysine 4 (04-745), BD Transduction Lab: p130 (610261), or Abcam: KDM5A/RBP2 (ab65769). The normal mouse IgG control antibody (cat.no. 12- 371B) is a component of the EZ ChIP™ system (Upstate Cell Signaling Solutions, Lake Placid, NY). ChIP assays were performed as described (12).
RESULTS
Induction of alkaline phosphatase
Alpl is induced early in osteoblast differentiation concomitantly with differentiation-associated cell cycle arrest. In an earlier study, stable expression of E1A in MC3T3-E1 pre-osteoblasts severely impaired induction of Alpl and blocked differentiation to the mineralization phenotype (4). The separate contributions of the pRB and p300 families were assessed here in lines stably expressing appropriate E1A mutants (Fig. 1A). Northern blot analysis (Fig. 1B) shows induction of Alpl is greatly impaired by sequestration of either family. Impaired expression is also apparent at the level of enzyme activity in an in situ staining assay, again indicating the pRB family is actively required for proper induction of Alpl during osteoblast differentiation (Fig. 1C). BRG1- depletion in the same background likewise impairs Alpl induction, while BRM-depletion accelerates induction (Fig. 1D), consistent with their respective roles in activation vs repression of osteoblast-specific gene expression (12).
The pRB family and p300 associate directly with the Alpl promoter and are required for the BRM to BRG1 switch
Chromatin immunoprecipitation (ChIP) analysis (Fig. 2, upper panel) detects all three pRB family members in direct association with the promoter. Occupation by p130 (lane 5) correlates specifically with repression (day 0), implying that p130 is part of a repressor complex as has been described in cell cycle studies (18). In contrast, pRB and p107 occupy the promoter specifically during activation (days 4 and 7), and concurrent with association of the global co-activator p300. (CBP was not detected although the antibody scored positive in corresponding controls, not shown). Parallel ChIP analysis reveals that Alpl is also a direct target of SWI/SNF complexes (lanes 8 and 9), and that the occupying complex changes from a BRM-containing configuration on the repressed promoter to a BRG1-containing configuration on the activated promoter.
Figure 2. Switch from BRM-specific to BRG1-specific SWI/SNF.
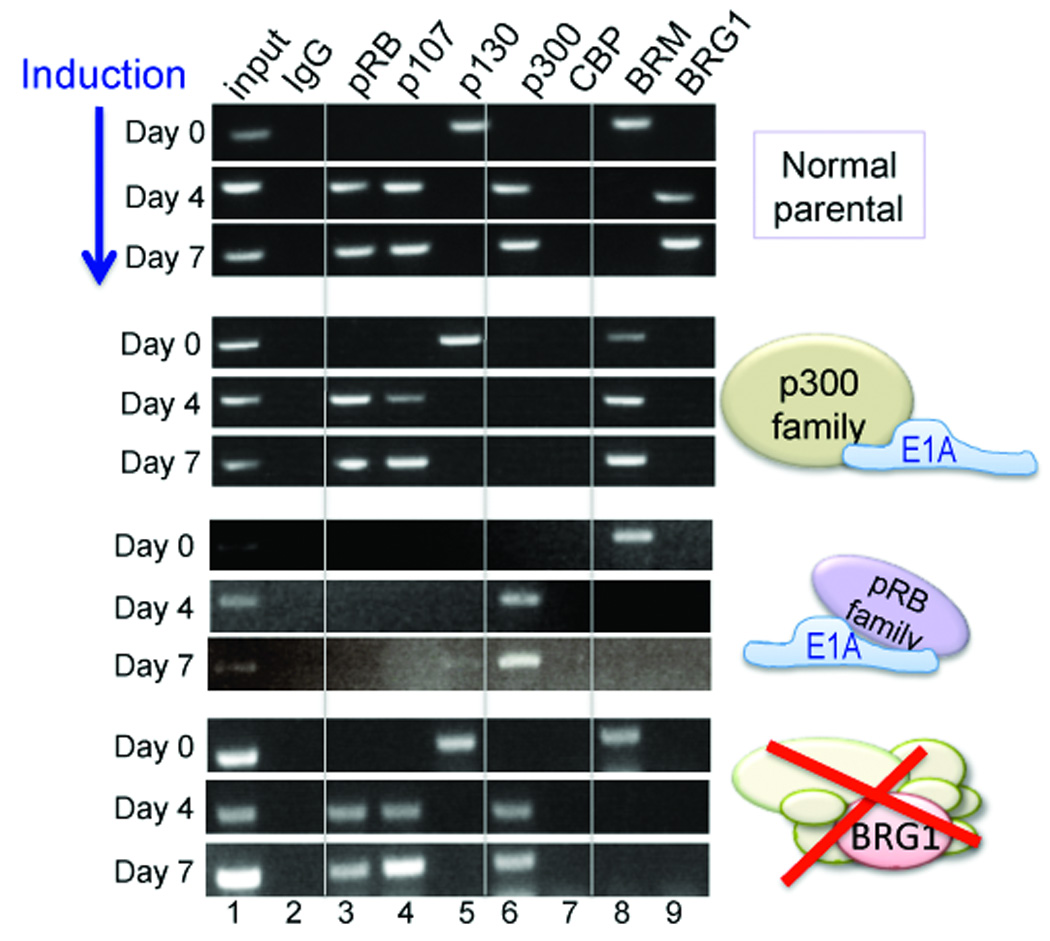
ChIP assays in normal parental cells show the timing of Alpl occupation by pRB family members, and by p300 and the alternative SWI/SNF complexes. Successive panels from the top show the promoter occupation dynamics in cell lines where the p300 family is sequestered, the pRB family is sequestered, or BRG1 is depleted.
Corresponding analysis in cells expressing the E1A variants shows a precise interplay between the E1A targets and the two forms of SWI/SNF. When the p300 family is targeted (Fig. 2, second panel), all the pRB family proteins associate with the promoter at the appropriate times, but BRM fails to dissociate. In contrast, when the pRB family is targeted (Fig. 2, third panel), p300 associates normally, and BRM dissociates successfully, but the activating BRG1-containing SWI/SNF configuration fails to occupy the promoter. Recruitment of BRG1 and pRB/p107 is not mutually dependent, as promoter occupation by pRB/p107 is successful in a BRG1-depleted line (Fig. 2, bottom panel). This panel also shows that BRM dissociation is independent of BRG1, meaning that displacement does not require direct competition from BRG1-containing complexes. These dynamics reveal an ordered two-step process in induction of Alpl, in which p300 is required for dissociation of a repressing BRM-specific form of SWI/SNF, and pRB/p107 is required for recruitment of an activating BRG1-specific form of SWI/SNF.
BRG1 is required for recruitment of RNA polymerase-II
ChIP analysis was also used to probe the association dynamics of basal transcription factors (Fig. 3A, top panel). TFIIB and TFIID are present even on the non-induced Alpl promoter in parental cells, but detection of RNA polymerase-II (pol-II) correlates with activation, and fails in the BRG1-depleted cell line (second panel). Whatever priming steps may be possible in the absence of BRG1, the crucial step of RNA polymerase recruitment depends substantially on the integrity of BRG1-specific complexes. BRG1-specific SWI/SNF recruitment fails when either family of E1A targets is subverted, and thus RNA polymerase recruitment fails as well (third and fourth panels).
Figure 3. Recruitment of RNA polymerase-II and dissociation of KDM5A.
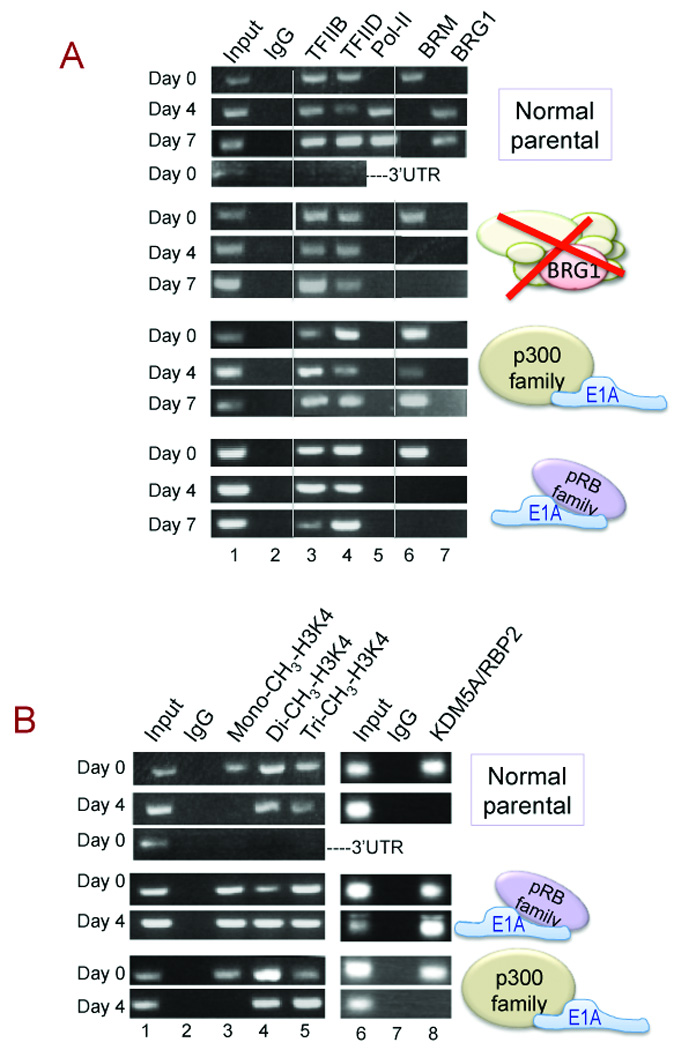
A. ChIP analysis shows the timing of Alpl occupation by basal transcription factors in normal parental cells, compared with lines in which BRG1 is depleted or the E1A targets are sequestered. Primers corresponding to the 3’UTR of Alpl were included as a negative control in parental cells where promoter occupation was positive throughout the time course.
B. ChIP analysis shows the methylation status of H3K4 and the promoter occupation dynamics of KDM5A/RBP2 on Alpl during differentiation in normal parental cells, compared with lines in which the E1A targets are sequestered. A 3’UTR control was included as described in panel A.
pRB family function is required for hyper-methylation of H3K4
An important event characteristic of activated promoters is tri-methylation at lysine-4 of histone H3 (H3K4) (19). Hyper-methylation of Alpl can be seen in normal cells as the methylation pattern shifts from one that includes the mono-methylated form at day 0 to a pattern in which only di- and tri-methylated forms are detectable at day 4 (Fig. 3B, lanes 3 to 5). A potential role for pRB in favoring the activated, hyper-methylated, state derives from recent identification of the pRB-targeted protein RBP2 (now designated KDM5A) as a histone demethylase that specifically removes a methyl group from di- or tri-methylated H3K4 (19). Exogenously expressed pRB can displace KDM5A/RBP2 from the osteocalcin promoter in pRB-null osteosarcoma cells to relieve repression and promote differentiation (10). These findings combine to predict that hyper-methylation at targeted promoters would be dependent on the pRB family. This is demonstrable on Alpl, as the mono-methylated form of H3K4 persists post-induction when the pRB family is sequestered (Fig. 3B, middle panel). Probing directly for the presence of KDM5A shows the demethylase does indeed occupy the repressed Alpl promoter (lane 8), and dissociates concurrent with activation and consistent with the shift in methylation pattern. When the pRB family is sequestered, KDM5A fails to dissociate. Sequestering the p300 family has no discernible effect on the methylase or the methylation pattern (Fig. 3B, lower panel).
Specific requirement for pRB
E1A is a valuable physiological probe, but it does not distinguish between individual members of the pRB family. To address the role of pRB specifically, shRNA was used to generate MC3T3-E1 lines stably depleted of pRB. Three independent lines were isolated, using two separate RNA-targeting sequences. Western Blots show pRB depletion in each line is specific with respect to p107 (Fig. 4A). An in situ staining assay shows severely impaired induction of alkaline phosphatase in each line (Fig. 4B); thus p107 is not sufficient in the absence of pRB to support Alpl activation. A ChIP assay performed in a representative line (Fig. 4C) shows successful p107 association with Alpl, but no detectable pRB on the promoter in response to the induction signal (lanes 3 and 4). Depletion of pRB results in failure to displace KDM5A and persistence of H3K4 mono-methylation (lanes 5 and 6), as well as failure to recruit BRG1 and RNA polymerase-II (lanes 9 and 11). Thus, whatever the contribution of p107, each of these promoter events requires pRB specifically.
Figure 4. pRB-specific effects.
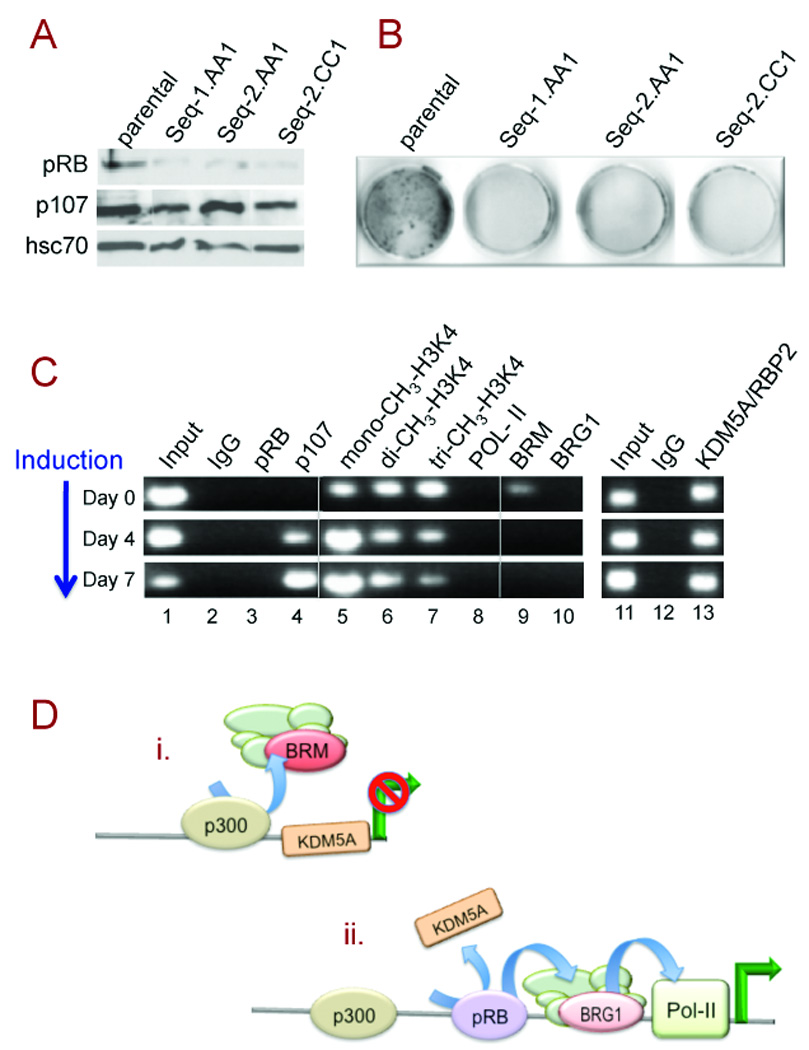
A. Western blot screening of three MC3T3-E1-derived lines stably expressing either of two shRNA sequences targeting pRB. The constitutive protein hsc70 is a loading control.
B. Staining at day-6 post-induction shows impaired induction of alkaline phosphatase in the pRBdepleted lines.
C. ChIP analysis in the Seq1.AA1 line. Depletion of pRB results in failure to displace KDM5A, persistence of H3K4 mono-methylation, and failure to recruit BRG1 and RNA polymerase-II.
D. Schematic illustration of the roles of p300 and pRB during the switch from: (i) the repressed state characterized by BRM-specific SWI/SNF occupation and the presence of KDM5A, to: (ii) the activated state characterized by displacement of KDM5A, and recruitment of BRG1-containing SWI/SNF. The unlabeled circles represent the non-enzymatic components of the SWI/SNF complex.
DISCUSSION
The transcriptional activation function of pRB has emerged as a key factor in understanding the genesis of osteosarcoma (1, 6, 7). An important new aspect of transactivation by pRB reported here is its essential role in recruitment of BRG1-containing SWI/SNF complexes to a target promoter. The respective roles of p300 and pRB in orchestrating the switch from BRM to BRG1 on Alpl are illustrated schematically in Fig. 4D. Displacement of BRM is dependent on p300 and provides the opportunity for pRB-dependent recruitment of the activating SWI/SNF complex containing BRG1. BRG1, like KDM5A, contains a canonical pRB-binding motif, LxCxE, and can partner directly with pRB (13, 20). BRG1-specific SWI/SNF is required in all tissue types for activation of key tissue-specific genes, and is essential for the ultimate recruitment of RNA polymerase-II to enact transcription on Alpl. These properties imply that recruitment of activating SWI/SNF complexes is a central component of transcriptional activation by pRB.
Osteocalcin is the best-studied model of osteoblast specific gene expression, due to its tightly regulated control and high level of induction, which remain apparent even in tumor cell models. The ability of exogenous pRB to displace KDM5A/RBP2 from the osteocalcin promoter in osteosarcoma cells revealed repressor competition as a significant aspect of pRB-mediated transactivation (10, 20). Importantly, the current findings reveal the same mechanism operating dynamically during activation of Alpl in normally differentiating osteoblasts. Consistent with subsequent recognition of the H3K4 demethylase activity of KDM5A (19), ChIP assays show directly that an activating shift in the H3K4 methylation pattern of Alpl is dependent on pRB.
Only a limited number of direct promoter targets of pRB-dependent gene activation have been identified to date, but the correlation between direct targeting and an activation function is strong in osteoblasts. In contrast, evidence for direct targeting of cell cycle genes by pRB during repression is conflicting, and some models favor indirect mechanisms of repression (2,3). Historically, pRB function has mostly been studied in epithelial cells, the natural hosts of the DNA tumor viruses that first linked the pRB family with cell cycle repression. Nevertheless, epithelial cell models, where pRB is less associated with tissue-specific gene activation, may have obscured the more important activation role of pRB in tumor susceptibility.
Other direct promoter targets of SWI/SNF in osteoblasts are emerging (12), and as these become more fully characterized it will be interesting to know whether pRB contributes to the activation of these genes as well. Regardless, Alpl is a highly significant target because the enzyme product is critical for terminal differentiation and because the timing of activation is closely linked with differentiationdependent cell cycle arrest. While malignant osteosarcoma cells may go on to express alkaline phosphatase, the transition towards terminal differentiation is the most likely point at which deficiency of pRB leaves osteoblasts susceptible to tumorigenesis (1, 6). Further study of the extent and dynamics of gene targeting by pRB in osteoblasts and related lineages will likely continue to increase understanding of the tumor profile associated with human germ-line mutation of Rb1.
Acknowledgments
This work was supported by PHS grants GM073257 (EM) and T32-CA134268 (SF).
REFERENCES
- 1.Tang N, Song WX, Luo J, Haydon RC, He TC. Osteosarcoma development and stem cell differentiation. Clin Orthop Relat Res. 2008;466:2114–2130. doi: 10.1007/s11999-008-0335-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burkhart D, Sage J. Cellular mechanisms of tumor suppression by the retinoblastoma gene. Nature Reviews Cancer. 2008;8:671–682. doi: 10.1038/nrc2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rizzolio F, Esposito L, Muresu D, Fratamico R, Jaraha R, Caprioli GV, Giordano A. RB gene family: genome-wide ChIP approaches could open undiscovered roads. J Cell Biochem. 2010;109:839–843. doi: 10.1002/jcb.22448. [DOI] [PubMed] [Google Scholar]
- 4.Beck GR, Jr, Sullivan EC, Moran E, Zerler B. Relationship between alkaline phosphatase levels, osteopontin expression and mineralization in differentiating MC3T3-E1 osteoblasts. J Cell Biochem. 1998;68:269–280. doi: 10.1002/(sici)1097-4644(19980201)68:2<269::aid-jcb13>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 5.Thomas DM, Carty SA, Piscopo DM, Lee JS, Wang WF, Forrester WC, Hinds PW. The retinoblastoma protein acts as a transcriptional coactivator required for osteogenic differentiation. Mol Cell. 2001;8:303–316. doi: 10.1016/s1097-2765(01)00327-6. [DOI] [PubMed] [Google Scholar]
- 6.Gutierrez GM, Kong E, Sabbagh Y, Brown NE, Lee JS, Demay MB, Thomas DM, Hinds PW. Impaired bone development and increased mesenchymal progenitor cells in calvaria of RB1 −/− mice. Proc Natl Acad Sci USA. 2008;105:18402–18407. doi: 10.1073/pnas.0805925105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berman SD, Yuan TL, Miller ES, Lee EY, Caron A, Lees JA. The retinoblastoma protein tumor suppressor is important for appropriate osteoblast differentiation and bone development. Mol Cancer Res. 2008;6:1440–1451. doi: 10.1158/1541-7786.MCR-08-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee JS, Thomas DM, Gutierrez G, Carty SA, Yanagawa S, Hinds PW. HES1 cooperates with pRb to activate RUNX2-dependent transcription. J Bone Miner Res. 2006;21:921–933. doi: 10.1359/jbmr.060303. [DOI] [PubMed] [Google Scholar]
- 9.Luan Y, Yu XP, Xu K, Ding B, Yu J, Huang Y, Yang N, Lengyel P, Di Cesare PE, Liu CJ. The retinoblastoma protein is an essential mediator of osteogenesis that links the p204 protein to the Cbfa1 transcription factor thereby increasing its activity. J Biol Chem. 2007;282:16860–16870. doi: 10.1074/jbc.M610943200. [DOI] [PubMed] [Google Scholar]
- 10.Benevolenskaya EV, Murray HL, Branton P, Young RA, Kaelin WG., Jr Binding of pRB to the PHD protein RBP2 promotes cellular differentiation. Mol Cell. 2005;18:623–635. doi: 10.1016/j.molcel.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 11.Reisman D, Glaros S, Thompson EA. The SWI/SNF complex and cancer. Oncogene. 2009;28:1653–1668. doi: 10.1038/onc.2009.4. [DOI] [PubMed] [Google Scholar]
- 12.Flowers S, Nagl NG, Jr, Beck GR, Jr, Moran E. Antagonistic roles for BRM and BRG1 SWI/SNF complexes in differentiation. J Biol Chem. 2009;284:10067–10075. doi: 10.1074/jbc.M808782200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cobrinik D. Pocket proteins and cell cycle control. Oncogene. 2005;24:2796–2809. doi: 10.1038/sj.onc.1208619. [DOI] [PubMed] [Google Scholar]
- 14.Stein GS, Lian JB, Stein JL, Van Wijnen J, Montecino M. Transcriptional control of osteoblast growth and differentiation. Physiol. Rev. 1996;76:593–629. doi: 10.1152/physrev.1996.76.2.593. [DOI] [PubMed] [Google Scholar]
- 15.Beck GR, Jr, Zerler B, Moran E. Gene array analysis of osteoblast differentiation. Cell Growth Differ. 2001;12:61–83. [PubMed] [Google Scholar]
- 16.Terao M, Studer M, Gianni M, Garattini E. Isolation and characterization of the mouse liver/bone/kidney-type alkaline phosphatase gene. Biochem. 1990;268:641–648. doi: 10.1042/bj2680641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hassan MQ, Tare R, Lee SH, Mandeville M, Weiner B, Montecino M, van Wijnen AJ, Stein JL, Stein GS, Lian JB. HOXA10 Controls Osteoblastogenesis by Directly Activating Bone Regulatory and Phenotypic Genes. Mol Cell Biol. 2007;27:3337–3352. doi: 10.1128/MCB.01544-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Litovchick L, Sadasivam S, Florens L, Zhu X, Swanson SK, Velmurugan S, Chen R, Washburn MP, Liu XS, DeCaprio JA. Evolutionarily conserved multisubunit RBL2/p130 and E2F4 protein complex represses human cell cycle-dependent genes in quiescence. Mol Cell. 2007;26:539–551. doi: 10.1016/j.molcel.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 19.Mosammaparast N, Shi Y. Reversal of histone methylation: biochemical and molecular mechanisms of histone demethylases. Annu. Rev. Biochem. 2010;79:155–179. doi: 10.1146/annurev.biochem.78.070907.103946. [DOI] [PubMed] [Google Scholar]
- 20.Gutierrez GM, Kong E, Hinds PW. Master or slave: the complex relationship of RBP2 and pRb. Cancer Cell (Previews) 2005;7:501–502. doi: 10.1016/j.ccr.2005.05.021. [DOI] [PubMed] [Google Scholar]


