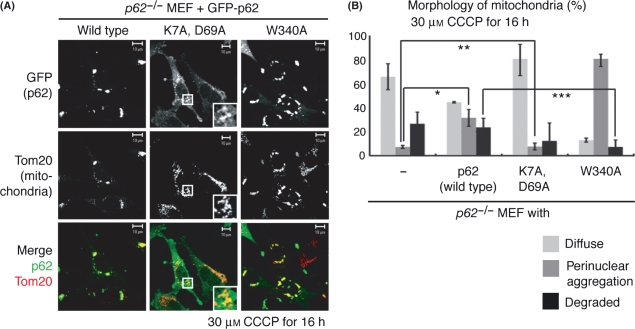
Figure 7.
The PB1 domain of p62 is imperative for the clustering of depolarized mitochondria. (A) Immunocytochemistry of p62−/− mouse embryonic fibroblasts (MEFs) complemented by wild-type p62 or the indicated p62 mutants. Higher magnification views of the boxed areas are shown in the insets in the middle panel. (B) The mitochondrial morphology of p62−/− MEFs expressing the indicated p62 mutants was analyzed in more than 100 cells per mutation. Bars represent the mean ± SD values of at least three experiments. Asterisk shows significant difference from control (P < 0.01; Student’s t-test) whereas two asterisks show no significance (P = 0.43 > 0.01), meaning that wild-type p62 restored the mitochondrial clustering, whereas the PB1 domain mutant (K7A, D69A) did not. Triple asterisks show significant difference from wild-type control (P = 0.005 < 0.01), meaning that mitochondrial degradation was impaired in p62−/− MEFs complemented with the p62 W340A mutant.