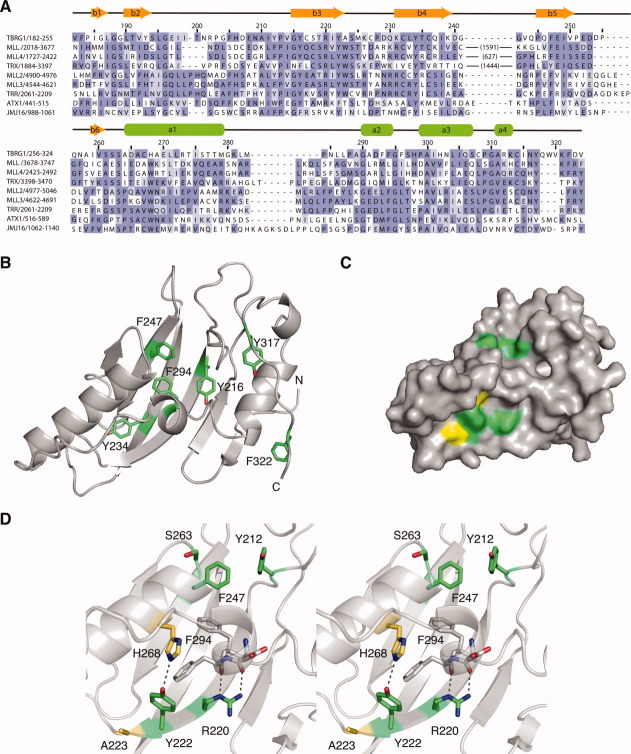
Figure 3.
Sequence conservation in FYR domains. A: Structure-based alignment of the sequences of selected FYR domains. The large insertions in MLL and related proteins are indicated in parentheses. Residues are colored based on degree of sequence conservation. B: Ribbon representation of the TBRG1 FYR domain showing the position of conserved phenylalanine and tyrosine residues in the hydrophobic core of the domain in green. C: Surface representation of the TBRG1 FYR domain highlighting conserved solvent-exposed residues. Highly conserved residues are colored green. Residues equivalent to the tryptophan residues conserved in chromatin-associated FYR domains are colored yellow. D: Stereo view of the two conserved surfaced patches. Hydrogen bonds mediated via the side chains of R220 and Y222 are highlighted with a black dashed line. The color-coding is the same as in panel (C).