Figure 5.
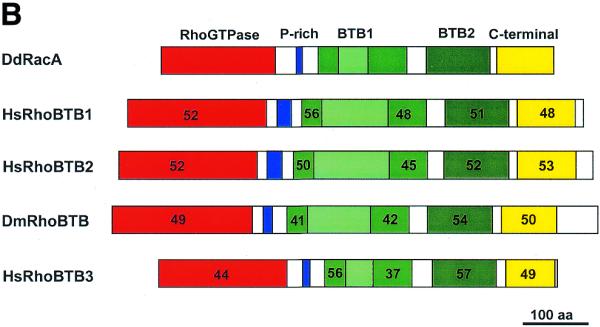
(Opposite and above) Multiple alignment and sequence comparison of RhoBTB proteins. (A) Multiple alignment and domain structure of RhoBTB proteins. A search of GenBank databases with the 400 residues long C-terminal part of DdRacA led to the identification of the complete sequence of one orthologue in Drosophila and three in human. Proteins of this novel subfamily are composed of a GTPase domain, a proline-rich region, two BTB domains (the first one divided into two subdomains by intervening sequences; dashed line) and a C-terminal domain of unknown function. Note that only HsRhoBTB3 ends with a prenylation signal. Sequences were aligned with ClustalX. Dashes indicate gaps introduced for optimal alignment. Residues identical or similar in at least three sequences appear in a black or grey background, respectively. Accession numbers are as follows: DmRhoBTB, AF217287; HsRhoBTB1, KIAA0740; HsRhoBTB3, KIAA0717; HsRhoBTB3, KIAA0878. (B) Comparison of DdRacA with other RhoBTB proteins. The numbers in each corresponding-colour region represent the percent similarity of each major domain of RacA to any of the other RhoBTB proteins. For the first BTB domain both subdomains have been calculated separately.

