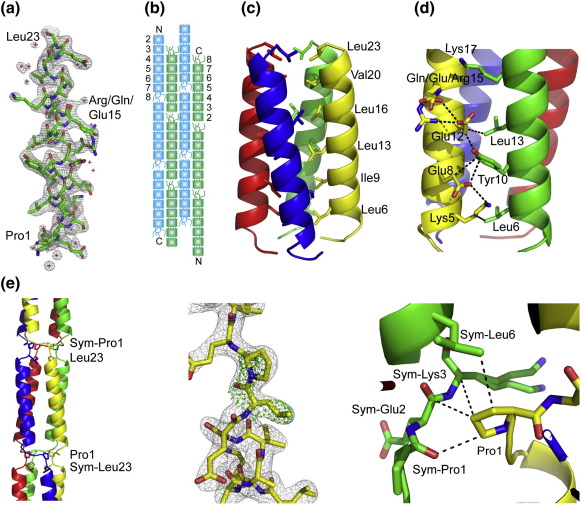
Fig. 2.
Structure of the 23-amino-acid repeat motif. (a) The 23-amino-acid model in the asymmetric unit shown with the final 2Fo − Fc electron density map (1.0σ contour). (b) Proposed packing of eight neck repeats in the crystals, with molecules running in opposite directions illustrated in blue and green. (c) Four-α-helical bundle formed by the 4-fold symmetry of the space group. The protein is shown in cartoon representation, with side chains of hydrophobic residues positioned toward the center of the bundle shown in stick representation. (d) Representative interactions between side chains in the four-helix bundle. (e) Connections between 23-amino-acid repeat motifs through non-helical segments. Left, one repeat is shown connected at the N- and C-termini to symmetry-related repeats. Center, close-up of the Leu23–Pro1 connection between successive repeats. The 2Fo − Fc electron density map (1.0σ contour) is shown as a gray mesh, and an Fo − Fc map made by omitting residues Pro1 and Leu23 from the model is shown in green (3.0σ contour). Right, interactions of Pro1 with symmetry-related monomers. All molecular graphic figures were prepared with PyMol (http://www.pymol.org).