Abstract
Gunnera plants have the unique ability to form endosymbioses with N2-fixing cyanobacteria, primarily Nostoc. Cyanobacteria enter Gunnera through transiently active mucilage-secreting glands on stems. We took advantage of the nitrogen (N)-limitation-induced gland development in Gunnera manicata to identify factors that may enable plant tissue to attract and maintain cyanobacteria colonies. Cortical cells in stems of N-stressed Gunnera plants were found to accumulate a copious amount of starch, while starch in the neighboring mature glands was nearly undetectable. Instead, mature glands accumulated millimolar concentrations of glucose (Glc) and fructose (Fru). Successful colonization by Nostoc drastically reduced sugar accumulation in the surrounding tissue. Consistent with the abundance of Glc and Fru in the gland prior to Nostoc colonization, genes encoding key enzymes for sucrose and starch hydrolysis (e.g. cell wall invertase, α-amylase, and starch phosphorylase) were expressed at higher levels in stem segments with glands than those without. In contrast, soluble sugars were barely detectable in mucilage freshly secreted from glands. Different sugars affected Nostoc’s ability to differentiate motile hormogonia in a manner consistent with their locations. Galactose and arabinose, the predominant constituents of polysaccharides in the mucilage, had little or no inhibitory effect on hormogonia differentiation. On the other hand, soluble sugars that accumulated in gland tissue, namely sucrose, Glc, and Fru, inhibited hormogonia differentiation and enhanced vegetative growth. Results from this study suggest that, in an N-limited environment, mature Gunnera stem glands may employ different soluble sugars to attract Nostoc and, once the cyanobacteria are internalized, to maintain them in the N2-fixing vegetative state.
Nitrogen (N) is an essential element for plant growth, but availability of reduced N in the soil is often limiting. Representatives from a wide range of land plants have evolved the ability to form associations with N2-fixing microbes (Franche et al., 2009). Associations between rhizobia and legume plants are well-characterized examples of plant-bacterial N2-fixing symbioses. Unlike rhizobia, which generally exhibit narrow host ranges (Kistner and Parniske, 2002), N2-fixing cyanobacteria are able to form productive associations with a broad range of plants, including bryophytes (hornworts and liverworts), ferns (Azolla), gymnosperms (cycads), and angiosperms (Gunnera; for review, see Rai et al., 2000; Adams et al., 2006). Free-living cyanobacteria within the genus Nostoc can fix N in specialized microoxic cells called heterocysts. The ability of Nostoc to fix N independent of a host environment may facilitate the formation of symbioses with a wide range of plants. Understanding the physiological conditions that enable a plant host to enter into symbiotic associations with cyanobacteria may allow us to extend the benefit of biological N fixation to crops outside the legume family.
Nostoc has the ability to differentiate not only into filaments bearing heterocysts but also into transiently motile filaments, known as hormogonia, which enable the cyanobacteria to enter plants (Meeks and Elhai, 2002). Nostoc can be induced to form hormogonia by different environmental stimuli and by a hormogonia-inducing factor released from N-stressed host plants (Meeks and Elhai, 2002; Adams et al., 2006). The attraction of hormogonia to plants is much less specific than that of rhizobia. Hormogonia are attracted to root extracts from either host or nonhost plants and even to certain simple sugars, such as Ara, Glc, and Gal (Nilsson et al., 2006). After entering a plant host, hormogonia revert back to filaments with N2-fixing heterocysts. Inside the host, further hormogonia formation is suppressed, and heterocysts appear at a frequency of about 30% to 40%, 3- to 4-times higher than that found in free-living Nostoc (Meeks and Elhai, 2002). Although free-living Nostoc species can support N2 fixation through photosynthesis, under symbiotic conditions they rely on photosynthate from the host plant. In general, the sugars (Suc, Glc, and Fru) known to support heterotrophic growth in the dark by free-living cyanobacteria coincide with those that support nitrogenase activity in Nostoc-plant associations (Meeks and Elhai, 2002). However, the Nostoc-Gunnera association may be exceptional; only Glc and Fru have been shown to sustain nitrogenase activities (Man and Silvester, 1994; Wouters et al., 2000), although Suc anddextrin were able to keep Nostoc alive without light (Wouters et al., 2000). It is evident from cyanobacterial studies that the plant hosts have evolved the ability to regulate cyanobacterial growth and differentiation during symbiotic associations (Meeks and Elhai, 2002).However, because most studies of plant-cyanobacterial associations have focused on the cyanobacterial partner (e.g. Wang et al., 2004; Ekman et al., 2006), the mechanisms through which plant hosts attract, internalize, and maintain cyanobacteria remain to be elucidated (Adams et al., 2006).
The Nostoc-Gunnera association is an ideal system with which to study plant-cyanobacteria symbioses, not only because Gunnera is the only genus of angiosperms known to form endosymbioses with N2-fixing cyanobacteria but also because the association between the two can be readily established in the laboratory (Bergman et al., 1992; Chiu et al., 2005). Nostoc hormogonia enter Gunnera plants through specialized glands located on the stem. As the gland matures, it secretes polysaccharide-rich mucilage that attracts cyanobacteria (Nilsson et al., 2006), supports their growth on the gland surface (Towata, 1985; Chiu et al., 2005), and permits further hormogonia differentiation (Rasmussen et al., 1994). From there, hormogonia enter the gland and penetrate cells near the base of the gland in the stem (Bonnett, 1990; Bergman et al., 1992). Although each gland is only transiently capable of accepting cyanobacteria, new glands continue to form on the stem at the base of each new leaf.
In contrast to the development of nodules in legumes, which requires a complex exchange of signals between the two symbiotic partners (Cooper, 2007), stem gland development in Gunnera takes place in the absence of cyanobacteria (Bonnett, 1990). N limitation, however, is a prerequisite for stem gland development (Chiu et al., 2005), as it is for nodulation (Barbulova et al., 2007). We have taken advantage of the N-deficiency-induced gland development in G. manicata to identify factors that enable Gunnera to form endosymbiosis with cyanobacteria. This study investigated changes in the carbohydrate metabolism during Gunnera gland development and discovered that tissue in the mature glands accumulated high levels of soluble sugars prior to the arrival of cyanobacteria. In agreement with this finding, several key genes encoding enzymes for starch/Suc hydrolysis were expressed at higher levels in the gland compared to the stem. Furthermore, we found that various sugars cyanobacteria may encounter as they approach Gunnera glands as opposed to those they would encounter within plant cells differentially affected Nostoc’s ability to form motile hormogonia.
RESULTS
Accumulation of Starch in Stems of N-Deprived G. manicata Seedlings
Glands on hypocotyls of G. manicata seedlings did not become mature when they were maintained on N-replete medium (Fig. 1A), consistent with a previous report (Chiu et al., 2005). In contrast, mature glands developed normally in plants grown on N-limited medium (Fig. 1, C and E). Internal structures of developing glands were analyzed by making cross sections through glands on hypocotyls. Staining fresh stem sections with KI/I2 revealed numerous starch-filled amyloplasts (black dots in Fig. 1, D and F) in the cortex of N-deprived seedlings but not in seedlings from N-replete medium (Fig. 1B). Although amyloplasts could also be found in cells within the mature glands on N-deprived seedlings, they were either less abundant (Fig. 1D) or greatly reduced in size (Fig. 1F) compared to those present in the cortex of the stem.
Figure 1.
Starch accumulation patterns in hypocotyls of G. manicata seedlings. A, An immature gland on the hypocotyl of a seedling grown on N-replete (N+) medium. B, A cross section through an immature gland as shown in A stained with KI/I2. C and E, Mature glands marked with anthocyanins on hypocotyls of seedlings from N-limited (N−) medium. D and F, Cross section of the stem through glands shown in C and E stained with KI/I2. Gland tissue is indicated with a g and vascular tissue at the center of the stem is indicated with a v. Note that starch-stained amyloplasts are mainly in the cortical cells but not in the gland.
Accumulation of Reducing Sugars in Stems of N-Deprived G. manicata Seedlings
The absence of starch granules in mature glands led us to wonder whether starch in the glands may be hydrolyzed to soluble sugars during gland maturation. To test this hypothesis, Benedict’s test for reducing sugars was performed on stem segments from Gunnera seedlings grown on sugar-free medium with (N+) or without (N−) combined N. Reducing sugars convert Cu2+ to Cu+, changing the blue color of Benedict’s solution to brick red cuprous oxide precipitate. Sugars that react with Benedict’s solution include Glc, Fru, Gal, Ara, and maltose. Homogenates of stem segments (without visible glands) or hypocotyls from seedlings grown on N+ medium retained most of the blue color of Benedict’s reagent (Fig. 2A, top row), indicating that there was little reducing sugars in these tissues. However, homogenates of stem segments from plants grown on N-free medium turned the Benedict’s reagent from blue to brown (Fig. 2A, bottom row), with the amount of brownish precipitate increasing as the number of glands increased. These results suggest that N-stressed Gunnera seedlings accumulate high levels of reducing sugars in the stem, especially in stems with glands.
Figure 2.
Comparison of reducing sugars in G. manicata seedlings using Benedict’s reagent. A, Top row, homogenates of stems (1 and 2) or a hypocotyl (3) from three different seedlings grown on N-replete (N+) medium. Bottom row, homogenates of stems with no gland (1) or with a single gland (2) or a hypocotyl with three glands (3) from seedlings grown on N-free (N−) medium. B, Estimated concentration of reducing sugars in glands versus stems of three soil-grown, N-stressed seedlings. Stems taken from each plant were divided into segments containing glands (Gland) or those lacking glands (Stem). After completion of the Benedict’s reaction, the A700 was measured for each sample. The concentration of reducing sugars in each sample was estimated according to a standard curve constructed using solutions with known concentrations of Glc.
The connection between gland development and the level of reducing sugars was assessed by comparing stem segments with and without glands from the same individual N-stressed soil-grown seedling. The estimated concentration of total reducing sugars in each sample varied greatly among the plants, with the smallest (no. 3) of the three plants containing the lowest amount of sugar (Fig. 2B). However, within the same plant, stem segments with glands had a consistently higher level of sugar than segments without glands (Fig. 2B). By comparing the A700 reading of each sample to a standard curve obtained using known concentrations of Glc, the concentration of total reducing sugars in stem segments without mature glands was estimated to range between 10 and 20 mm, and the concentration estimated in stem with mature glands was between 20 and 25 mm (Fig. 2B).
Accumulation of Monosaccharides in Tissue of Mature Glands
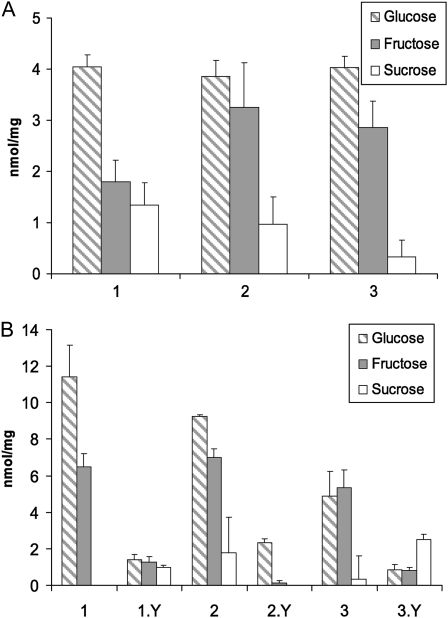
The identity and concentration of sugars in mature glands were elucidated by extracting soluble sugars from stem segments of N-deprived, soil-grown G. manicata seedlings (about 2 months old) possessing mature glands actively secreting mucilage. Quantitative analysis of Glc, Fru, and Suc was performed using the enzymatic NADP-reducing method (Bergmeyer and Bernt, 1974). As shown in Fig. 3A, around 4 nmol mg−1 fresh weight (4 mm) of Glc and between 2 to 3 nmol mg−1 fresh weight (2−3 mm) of Fru were detected in the three plants analyzed. Although Suc was detected in the samples, it was at a lower concentration (<1 nmol mg−1 fresh weight) than that of Glc or Fru. The combined concentration of Glc and Fru in these samples was around 7 mm, which is much lower than the concentration of total reducing sugars (20−25 mm) in the glands estimated using Benedict’s test. Due to the specificities of enzymes used in the NADP-reducing method, other reducing sugars in the sample could not be detected.
Figure 3.
Quantitative analysis of soluble sugars in stem segments containing mature glands from G. manicata seedlings. A, Concentrations of soluble sugars in three N-deprived, Nostoc-free soil-grown plants. B, Concentrations of soluble sugars in the stems of three Nostoc-colonized, soil-grown plants. Each pair (e.g. 1 and 1.Y) represents stem segments from one plant. Segments 1, 2, and 3 are from older, Nostoc-free portions of the stem. Segments 1.Y, 2.Y, and 3.Y are from younger, colonized portions of the stem. Note the difference in scale between A and B.
Soluble Sugar Accumulation in Nostoc-Colonized Plants
The effect of Nostoc colonization on sugar accumulation in the plant was analyzed by extracting soluble sugars from stem segments of soil-grown, N-deprived G. manicata seedlings (taken from the same batch as the ones used for Fig. 3A) about 1 month after Nostoc punctiforme inoculation. At the time of harvest, colonization of Nostoc on these plants was determined to be successful as judged by the greening of leaves. Stem sections revealed the presence of dark-green Nostoc colonies in the upper, younger half of the stems near the shoot tip but not in stems from the lower, older part of the plants. This was most likely due to the fact that glands on the lower part of the stem were no longer functioning at the time of Nostoc inoculation. As shown in Figure 3B, for all three plants analyzed, the concentrations of Glc and Fru in the uncolonized older stems were at least 4 times higher than those found in the Nostoc-colonized, younger stems from the same plant. The combined concentrations of Glc and Fru in the older stems ranged from 10 to 18 mm, which are much higher than the 7 mm obtained from stems of younger plants before they were inoculated with Nostoc (Fig. 3A). These results indicate that, under N-limited condition, sugar accumulation in the stem increased as the plant grew older. Furthermore, successful colonization by Nostoc, which resupplies the plant with fixed N, greatly reduced measureable free Glc and Fru in the surrounding stem tissue.
Detection of Soluble Sugars in the Mucilage
Mucilage from Gunnera glands contains polymers of Ara, Gal, and GlcUAs (Rasmussen et al., 1996). However, no information regarding soluble sugars in the mucilage has been reported. Soluble sugars in the mucilage were characterized using samples collected from the same Nostoc-free, soil-grown G. manicata plants used for the analysis of soluble sugars in the gland tissue (Fig. 3A). In sharp contrast to the high sugar levels within the gland, very low levels of Glc (0.03–0.04 nmol mg−1 fresh weight), Fru (0.01–0.02 nmol mg−1 fresh weight), and Suc (0.01 nmol mg−1 fresh weight) were detected in these mucilage samples. Overall, the concentration of sugars in the mucilage amounts to only about one-hundredth of that found in the mature glands from which the mucilage samples were collected (Fig. 3A).
Benedict’s test was also used to detect total reducing sugars present in mucilage collected from different batches of soil-grown, N-deprived G. manicata seedlings. In agreement with the low level of sugars detected in the above-mentioned analysis, reducing sugar levels in the mucilage collected from seedlings were insufficient to change the color of Benedict’s reagent (data not shown).
To see if older plants produce mucilage with higher sugar content, mucilage from shoot apex of several adult plants (2 years old) was collected and analyzed. Mucilage from these plants was almost gel like, thus an equal volume of water was needed to extract the soluble fraction for Benedict’s test. Slight changes in the color of Benedict’s reagent were detected in some samples; however, even in the best case, the A700 reading obtained from the mucilage extract translated to about 7 mm of total reducing sugars, which was still much lower than the estimated concentration in the gland tissue of Gunnera seedlings (Fig. 2B). These results suggest that Gunnera mucilage, especially mucilage from adult plants, may contain reducing sugars but only in limited amounts.
Expression of Genes Encoding Enzymes for the Hydrolysis of Complex Carbohydrates
The mechanism through which simple sugars accumulate in mature glands was investigated by analyzing genes encoding enzymes for starch and Suc hydrolysis identified in a normalized, gland-enriched cDNA library from G. manicata (W.-L. Chiu, E.K. Breathwaite, C.R. Secor, and J. Elhai, unpublished data). Sequences of cDNA library (obtained using 2nd-generation DNA sequencing technologies) were screened for genes of interest using the Kyoto Encyclopedia of Genes and Genomes database. The proteins encoded by each cDNA sequence were verified by comparing them to the nonredundant protein database via BLASTX (Altschul et al., 1997). Among cDNA sequences identified (see Table I) were those encoding enzymes that facilitate starch degradation, such as α-amylase (EC 3.2.1.1; an endoamylase that initiates starch degradation), β-amylase (EC 3.2.1.2; an exoamylase that converts amylose to maltose), and a starch phosphorylase (EC 2.4.1.1; an enzyme that catalyzes the reversible transfer of Glc1P to α-1,4-glucan chains). A homolog of STARCH-EXCESS 4 (SEX4), a phosphoglucan phosphatase required for starch degradation (Kötting et al., 2009), was also identified in the gland cDNA library.
Table I. RT-PCR primers for G. manicata cDNA encoding starch/Suc degradation-related proteins.
F, Forward primer; R, reverse primer.
| Annotation (GenBank No.) | Predicted Size (Region Covered)a | % Positive (Amino Acid)b | Primers for RT-PCR (5′ → 3′) |
| Invertasecell wall (HQ009838) | (103–574 amino acids) | 79 | F:GTCGCTAAGAACCTTGATAGATCAC |
| R:TCTAACCTCTCATAGGTTGGTTCAA | |||
| Invertasecytosolic (HQ009839) | 571 amino acids | 89 | F:GCCTGAATACTACGATGGAAAGCTA |
| R:ATCAACAAGTCCAAGACGCAGA | |||
| Suc synthase (HQ009840) | 811 amino acids | 93 | F:GGCGAGCTCTATCGTTACATAGC |
| R:CACCTGATCAGGGTGATACGGATC | |||
| α-Amylase (HQ009841) | 422 amino acids | 79 | F:GAGTTGTGGCGATTGAAGGATTCG |
| R:GAACAGTGACGGGATTCCAGGAT | |||
| β-Amylase (HQ009842) | 543 amino acids | 76 | F:TGTTGGATGATAACGAGGTAGTGG |
| R:GACTCAAGCTACTTGCAAGCTAAC | |||
| Starch phosphorylase (HQ009843) | (376–818 amino acids) | 80 | F:GGCTGCTGCGGATAATGCAT |
| R:TTCTGATGGGACGAGCAAGAG | |||
| SEX4 (HQ009844) | 381 amino acids | 92 | F:GCTCCCAACAAAGATGGTCATGTC |
| R:GATGTCCATGCGTTAAGAATACTCTC | |||
| Arabinofuranosidase (HQ009845) | 690 amino acids | 82 | F:CACTTCTACAGGATCATCAAAGACTG |
| R:GAAGATATAAGGCTGGAACCTGC | |||
| AGPfasciclin (HQ009846) | (24–404 amino acids) | 77 | F:GCATTCTCCAAAGGCATCGAA |
| R:CTAACGGCTGCGTTCTCGT | |||
| AGPclassical (HQ009847) | 201 amino acids | 91 | F:GCTCGTACCAATCTCGCC |
| R:ACTTTAGGACCTACATACTGTGGC | |||
| TIP41-like protein (HQ009848) | 287 amino acids | 82 | F:ATGGAGAGCCCATTATTCTTCGAG |
| R:CAGACTGATGATGGGAAGCCTC |
Coordinates in parentheses refer to those of the protein that best matches the incomplete cDNA sequences from G. manicata.
Percentage of positive amino acids compared to the best matched protein obtained through BLASTX.
Enzymes identified relating to Suc hydrolysis represented in the cDNA library include two invertases (EC 3.2.1.26), a cell wall invertase and a neutral (cytoplasmic) invertase, and a Suc synthase (EC 2.4.1.13; a glycosyltransferase that catalyzes the reversible cleavage of Suc to UDP-Glc and Fru). Also identified in the gland cDNA library was a cDNA encoding a homolog of Arabidopsis (Arabidopsis thaliana) ARAF1, an extracellular α-l-arabinofuronosidase (ARAf; EC 3.2.1.55) capable of catalyzing the hydrolysis of Ara from extracellular polysaccharides such as arabinogalactan-proteins (AGPs) or arabinan-containing pectins (Chávez Montes et al., 2008). The ARAF1 homolog is of interest because it may catalyze the release of Ara from the mucilage.
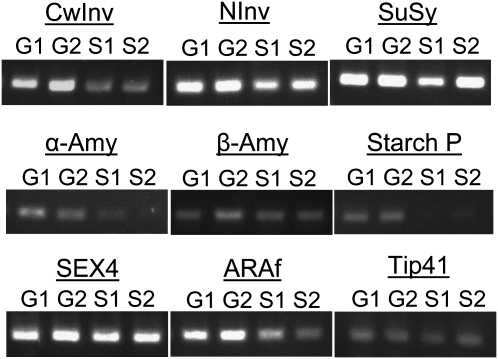
The expression levels of the above-mentioned genes in the gland and normal stem tissue were analyzed. Total RNA was extracted from stem segments with or without glands, taken from two different batches of G. manicata seedlings grown on N-free Murashige and Skoog medium. Reverse transcription (RT)-PCR was performed, using the gene-specific primers listed in Table I. A homolog of type 2A phosphatase activator (TIP41-like protein) was used as an internal control (Expósito-Rodríguez et al., 2008). In general, genes for enzymes involved in the hydrolysis of Suc (Fig. 4, top panel) seemed to be expressed at higher levels than those involved in the breakdown of starch (Fig. 4, middle panel). The high levels of Suc hydrolytic enzymes may explain why N-deprived Gunnera plants accumulate mainly Glc and Fru with only a low level of Suc (Fig. 3). Among genes for Suc hydrolytic enzymes, cell wall invertase was expressed at a higher level in stem segments with glands compared to those without. However, both neutral invertase and Suc synthase were expressed at high levels in the stem segments with or without the glands (Fig. 4, top panel).
Figure 4.
RT-PCR comparison of gene expression in stem segments of N-deprived G. manicata seedlings. Expression levels of genes encoding enzymes for the hydrolysis of starch and Suc in stem segments with glands (G1 and G2) and those without glands (S1 and S2) were compared by two-step RT-PCR using total RNA from two different batches of G. manicata seedlings grown on N-deprived medium. Abbreviations: CwInv, cell wall invertase; NInv, neutral invertase; SuSy, Suc synthase; α-Amy, α-amylase; β-Amy, β-amylase; Starch P, starch phosphorylase. Tip41 was used as an internal control. Due to the very high expression levels for NInv and SuSy, only 3 μL instead of 5 μL of PCR product for these two genes was loaded on the gel.
Among genes encoding enzymes for starch hydrolysis, the expression levels for α-amylase and starch phosphorylase were higher in stem segments with glands compared to those without, whereas β-amylase expression was similar with or without the glands (Fig. 4, middle panel). SEX4, a potential regulator for starch degradation, showed no differences in the expression level regardless of the glands (Fig. 4, bottom panel).
The expression level of ARAf, the extracellular enzyme that may release Ara from AGP or pectin in the mucilage, was slightly higher in the stem segments containing glands than those without (Fig. 4, bottom panel), although the expression level of two genes for AGP core proteins, one classical AGP and one fasciclin-like AGP (Seifert and Roberts, 2007), were the same regardless of the glands (data not shown). However, since the gland sample also contained the neighboring stem tissue, the actual differences in the gene expression levels between stem segments with and without glands are likely underestimated.
Effects of Soluble Sugars on Nostoc Differentiation
The initial contact between Nostoc and Gunnera takes place in the mucilage, which promotes the differentiation of motile hormogonia (Rasmussen et al., 1996). However, once Nostoc hormogonia enter Gunnera cells, further hormogonia differentiation does not take place. Instead, filaments revert to vegetative cells interspersed with heterocysts. The dramatic differences in the levels of soluble sugars between mucilage and the gland tissue prompted us to test whether soluble sugars can influence Nostoc differentiation. In the mucilage, hormogonia differentiation could conceivably be affected by sugars, such as Ara and Gal, released from the hypothetical hydrolysis of AGPs (Rasmussen et al., 1996). As cyanobacteria enter cells of the mature glands, they may encounter Glc, Fru, and Suc. Accordingly, we tested the effect of these soluble sugars on Nostoc hormogonia differentiation by streaking a dense culture of N. punctiforme onto fresh N-free solid medium supplemented with 30 mm of Ara, Gal, Glc, Fru, Suc, or no sugar.
Upon transfer to fresh N-free media, Nostoc filaments differentiated into hormogonia after overnight incubation, regardless of the type of sugar present. They remained as hormogonia for 3 d before reverting back to the N2-fixing state. After day 3, however, filaments on the Suc plates remained in the vegetative state until day 20, but Nostoc on the remaining plates reentered the hormogonia formation cycle by day 7. Subsequently, the extent of hormogonia formation was influenced by the specific sugar in the medium, as indicated by the reduced number of small rod-like hormogonia 3 weeks after the initial hormoglinia induction (Fig. 5, top panels). Arabinose and sugar-free media had a similar effect on hormogonia formation. In both cases, the hormogonia formation cycle continued until the end of the experiment. In a separate batch of experiments, Fru was found to inhibit secondary hormogonia formation to the same extent as Glc (data not shown). Overall, the inhibitory effect of sugars on hormogonia differentiation was in the order of Suc > Glc/Fru > Gal > Ara. The measurements of hormogonia frequency were corroborated by observations of the density of growth on the medium 5 weeks later, which was affected by the presence of sugar in the same way as hormogonia differentiation (Fig. 5, bottom panels). Nostoc on medium with Ara or without sugar did not grow as well, presumably because frequent hormogonia formation prevented heterocyst differentiation required for growth on N-free medium.
Figure 5.
The effects of soluble sugars on N. punctiforme hormogonia differentiation. N. punctiforme was induced to form hormogonia (i.e. the rod-shaped filaments in the top panels) by streaking cells from a dense culture onto fresh N-free solid medium supplemented with 30 mm of the indicated sugar or with no sugar. Streaking was confined to the center of the plate, marked by a red circle (1 cm in diameter) visible in some bottom panels. Nostoc filaments differentiated into hormogonia after overnight incubation on fresh medium; however, the frequencies of subsequent hormogonia formation varied, depending on the specific sugar present in the media. Pictures of the culture were taken under a stereo microscope 3 weeks (top panels) or 5 weeks (bottom panels) after initial hormogonia induction. The magnifications of the top and bottom panels were approximately 64× and 7.5×, respectively. The red and blue dots on the bottom panels were used to mark the extent of hormogonia movement at the end of the first week.
DISCUSSION
To establish productive associations with N2-fixing cyabanobacteria, plant hosts must solve the problems of attracting and internalizing cyanobacteria and regulating cyanobacterial growth and differentiation once the cyanobionts are inside the plant. This study took advantage of the N-deprivation-induced gland development in G. manicata (Chiu et al., 2005) to identify factors that may enable Gunnera plants to establish endosymbiosis with Nostoc.
Similar to other plants under chronic N stress (Hermans et al., 2006; Bi et al., 2007), G. manicata seedlings grown on N-deprived medium accumulated a large amount of starch in the stem (Fig. 1). In contrast, cells in the mature glands were nearly starch free (Fig. 1, D and F). Instead, mature glands accumulated high levels of soluble sugars, especially Glc and Fru (Fig. 3A), compared to normal stem tissue (Fig. 2B). Glc and Fru have been shown to support nitrogenase activities in Nostoc strains associated with Anthoceros (Steinberg and Meeks, 1991) and Gunnera (Man and Silvester, 1994; Wouters et al., 2000). In stem segments with mature glands, the total concentration of Glc and Fru was around 7 mm (Fig. 3A). As the plants grew older, the level of these sugars in uncolonized stems continued to increase (Fig. 3B). However, the actual levels of soluble sugars may be even higher since the estimated concentration of total reducing sugars in stems with mature glands, using Benedict’s test (Fig. 2B), was between 20 to 25 mm. Hence, the level of metabolizable sugars in Gunnera stems prior to Nostoc colonization is likely higher than the 10 mm Fru used to sustain long-term nitrogenase activity in darkness for Nostoc PCC 9229, a strain originally isolated from Gunnera monoica (Wouters et al., 2000). Successful Nostoc colonization drastically reduced Glc and Fru accumulation in the colonized portion of the stem (Fig. 3B), presumably because most sugars were consumed to support N2 fixation and amino acid synthesis. This is consistent with the observation that Gunnera plants grown on N-replete medium did not accumulate reducing sugars (Fig. 2A).
As one would expect from the high Glc and Fru levels in N-stressed G. manicata plants (Fig. 3), several genes encoding Suc-cleaving enzymes, including cell wall invertase, neutral (cytoplasmic) invertase, and Suc synthase, were expressed at very high levels in the stem, especially the latter two (Fig. 4, top panel). Expression of genes encoding enzymes for starch degradation could also be detected, but their RNA was not as abundant compared to those for Suc hydrolysis (compare Fig. 4 top and middle panels). Starch phosphorylase and α-amylase were expressed at higher levels in the stem segments with glands (Fig. 4, middle panel), which may be partially responsible for the lack of starch accumulation in the glands. Increased starch and Suc breakdown through higher expression of starch and sugar degradation enzymes were also observed in rhizobium-colonized nodules (Colebatch et al., 2004; Flemetakis et al., 2006; Tesfaye et al., 2006). Identifying the mechanisms through which plants regulate expression of genes for starch and Suc hydrolysis enzymes in the symbiotic tissue is crucial to understand the ability of the plant tissue to host N2-fixing microbes.
Despite high levels of soluble sugars in gland tissue, sugar levels in the mucilage collected on the gland surface were barely detectable. Earlier analysis of mucilage from Gunnera chilensis indicated that carbohydrates in the mucilage are mainly polymers of Ara, Gal, and GlcUA in the ratio of 1.00:0.25:0.13 (Rasmussen et al., 1996). The same study suggested that polysaccharides in the Gunnera mucilage are mainly arabinogalactans associated with AGPs (Rasmussen et al., 1996). Through analysis of glycosidic linkage composition, AGPs were also implicated as the primary component of mucilage secreted by pea (Pisum sativum) roots (Knee et al., 2001). However, the ratio of Ara to Gal in legume root mucilage is close to 1:1 (Knee et al., 2001), quite different from the 4:1 ratio found in the Gunnera mucilage (Rasmussen et al., 1996). The higher Ara to Gal ratio may be due to arabinan-containing pectins in the Gunnera mucilage in addition to AGPs. Since breakdown of the middle lamella takes place in the mature glands (Towata, 1985), it is reasonable to have some remnant of pectin from the middle lamella appear in the mucilage. The Ara in the mucilage may be released through an ARAf encoded by a homolog of Arabidopsis gene ARAF1, which can use pectin as substrate (Chávez Montes et al., 2008). This ARAf is expressed at a higher level in stem segments containing glands (Fig. 4, bottom panel). The presence of Ara in the mucilage may be of functional significance because Ara was found to be more effective than Glc or Gal in attracting Nostoc hormogonia, although none of these simple sugars was as effective as root extract from white clover (Trifolium repens) or mucilage from G. manicata (Nilsson et al., 2006).
If free Ara and Gal in the mucilage is sufficient to attract Nostoc (Nilsson et al., 2006), they should not interfere with the Nostoc hormogonia formation cycles (Fig. 5). This is important because the ability to form hormogonia is required before Nostoc filaments enter plant cells. Once inside the Gunnera cells, however, hormogonia formation must be prevented for N2 fixation to take place. The abundance of Glc and Fru in tissue associated with mature glands may serve to inhibit hormogonia formation and promote Nostoc heterocyst differentiation and N2 fixation.
The inhibition of hormogonia formation by Glc and Fru may be mediated by the action of a water-soluble hormogonia repression factor similar to that produced by the hornwort Anthoceros punctatus (Meeks and Elhai, 2002). Hormogonia repression factor induces genes that repress hormogonia formation through inactivation of a transcriptional repressor HrmR (Campbell et al., 2003). HrmR belongs to the LacI/GalR family of sugar-binding repressors and binds galacturonate in vitro (Campbell et al., 2003). According to the model proposed by Campbell et al. (2003), HrmR would be sugar bound and inactive in the high sugar concentrations found inside of Gunnera cells, thus allowing genes that negatively regulate hormogonia formation to be expressed.
Taken together, results from this study suggest that, in an N-limiting environment, Gunnera glands secrete mucilage with very low levels of soluble sugars, which help to attract Nostoc to the surface of the gland but does not interfere with the hormogonia formation cycle. From there, hormogonia travel into the gland and enter cells at the base of the gland most likely because they are attracted by the high levels of soluble sugars inside these cells. Once inside plant cells, further hormogonia formation is prevented by the high levels of sugars in these cells. Understanding how the expression of genes encoding starch/Suc degradation enzymes is up-regulated in the tissue associated with mature glands will be crucial to understanding the ability of Gunnera tissue to provide an environment for the establishment of endosymbiosis with cyanobacteria.
MATERIALS AND METHODS
Cultivation of Nostoc Punctiforme
Nostoc punctiforme ATCC 29133 was obtained from Jack Meeks (University of California at Davis) and cultivated on N-free medium according to Chiu et al. (2005). To test the effect of various sugars on hormogonia formation, a small amount of N. punctiforme from a 3- to 4-week-old N-free plate-grown culture was streaked out onto the center (about 5 mm in diameter) of a 60 × 10 mm petri plate containing fresh N-free solid medium supplemented with 30 mm of one of the following sugars: Suc, Glc, Fru, Gal, or Ara. Prior to autoclaving, each medium was buffered with 0.5 mm MES (pH 5.8). All media were solidified with 0.6% (w/v) Gellan Gum (Caisson Laboratories). Cultures were maintained at 25°C under a 16-h/8-h photoperiod at 50 μmol m−2 s−1. The cultures were observed for 5 weeks with data recorded every other day. Pictures of the cultures were taken using an Olympus SZH stereo microscope.
Cultivation and Manipulation of Gunnera manicata Seedlings
Germination of Gunnera manicata seeds was as described by Chiu et al. (2005). Seedlings were maintained in 0.5× Murashige and Skoog medium supplemented with 0.5% (w/v) Suc. To induce gland development, seedlings were transferred to N-free Murashige and Skoog medium (PhytoTechnology Laboratories) without sugar and grown at 25°C under constant light at 30 μmol m−2 s−1. To induce gland development in soil-grown plants, plate-grown seedlings were transferred to 42 mm Jiffy-7 peat pellets (Jiffy Products), irrigated with tap water, and maintained in a growth chamber at 25°C under a 16-h/8-h photoperiod at 40 μmol m−2 s−1. Under this growth condition, glands begin producing mucilage after about 1 month.
Detection of Starch and Reducing Sugars in Gunnera Tissue
Starch was detected by adding a drop of a solution containing 1% w/v each of I2 and KI onto fresh sections of Gunnera seedling stems. For reducing sugar assays, approximately 30 mg of Gunnera tissue was weighed and frozen in liquid N. The samples were then ground up in 60 μL of Benedict’s reagent in a microfuge tube and incubated at 95°C for 5 min. The samples were cleared by centrifugation, and the remaining blue color of Cu2+ in Benedict’s reagent was quantified by measuring the absorption at 700 nm (A700; Cochran et al., 2008) using a NanoDrop spectrophotometer (Thermo Scientific). A700 reading for each sample was normalized against the sample weight. Since the disappearance of blue color depends on reducing sugars in the homogenate, the amount of reducing sugars in each sample is negatively correlated to A700. The concentration of total reducing sugars in each sample was estimated according to a standard curve constructed using known concentrations of Glc solutions.
Quantitative Analysis of Soluble Sugars in Gunnera Tissue
Stem segments of G. manicata were weighed, frozen in liquid N, and stored in liquid N until use. To extract soluble sugars, stem segments weighing between 100 and 200 mg were ground to a fine powder in liquid N using a mortar and pestle. The powder was suspended in 2.5 mL of 80% ethanol, and the mixture was heated to 80°C for 1 h with occasional vortexing. After a brief centrifugation, the supernatant was collected. The ethanol extraction procedure was repeated once. The supernatant was then dried in 100 μL aliquots using a SpeedVac concentrator (Thermo Scientific) and reconstituted in water. The concentrations of Glc, Fru, and Suc were measured using an enzymatic NADP-reducing method (UV method) kit from Roche based on Bergmeyer and Bernt (1974). In brief, Glc and Fru in the sample were first phosphorylated by hexokinase to yield Glc-6-P (G-6-P) and Fru-6-P. Subsequently, in the presence of G6P dehydrogenase, G-6-P was detected by its ability to reduce NADP+ to NADPH. Fru-6-P was converted to G-6-P by phosphoglucose isomerase and Suc was hydrolyzed to Fru and Glc by invertase before they were measured. The amount of NADPH produced was measured by reading the A340 using a NanoDrop spectrophotometer (Thermo Scientific).
RNA Isolation and RT-PCR Analysis of Gene Expression
Stems of G. manicata seedlings grown on N-free Murashige and Skoog medium with mature glands were divided into two segments, one with glands (the upper half) and one without glands (the lower half) and immediately frozen in liquid N. Total RNA was isolated from these samples using TriZol RNA reagent (Molecular Research Center). First-strand cDNA synthesis was carried out using SuperScript II reverse transcriptase (Invitrogen) according to manufacturer’s instructions. First-strand cDNA was diluted 5-fold and used as a template for PCR. PCR was carried out using GoTaq Green Master Mix (Promega) and gene-specific primers listed in Table I. The PCR conditions were 30 s at 94°C, 30 s at 55°C, 30 s at 72°C, and 5 min at 72°C for 35 cycles with 3 min at 94°C to start up.
Sequence data from this article can be found in the GenBank/EMBL data libraries under the accession numbers listed in Table I.
Acknowledgments
The authors would like to thank Yi-Fang Tsay, Sarah Weeda, and Gerald Peters for critical reading of this manuscript.
References
- Adams DG, Bergman B, Nierzwicki-Bauer SA, Rai AN, Schubler A. (2006) Cyanobacterial-plant symbioses. Dworkin M, Falkow S, Rosenberg E, Schleifer K-H, Stackebrandt E, , The Prokaryotes, Vol 1 Springer, New York, pp 331–363 [Google Scholar]
- Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25: 3389–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbulova A, Rogato A, D’Apuzzo E, Omrane S, Chiurazzi M. (2007) Differential effects of combined N sources on early steps of the Nod factor-dependent transduction pathway in Lotus japonicus. Mol Plant Microbe Interact 20: 994–1003 [DOI] [PubMed] [Google Scholar]
- Bergman B, Johansson C, Söderbäck E. (1992) The Nostoc-Gunnera symbiosis. New Phytol 122: 379–400 [DOI] [PubMed] [Google Scholar]
- Bergmeyer HU, Bernt E. (1974) Sucrose/D-glucose. Bergmeyer HU, , Methods of Enzymatic Analysis. Verlag Chemie, Weinheim/Academic Press, New York, pp 1176–1179 [Google Scholar]
- Bi YM, Wang RL, Zhu T, Rothstein SJ. (2007) Global transcription profiling reveals differential responses to chronic nitrogen stress and putative nitrogen regulatory components in Arabidopsis. BMC Genomics 8: 281–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnett HT. (1990) The Nostoc-Gunnera association. Rai AN, , Handbook of Symbiotic Cyanobacterium. CRC Press, Boca Raton, FL, pp 161–171 [Google Scholar]
- Campbell EL, Wong FCY, Meeks JC. (2003) DNA binding properties of the HrmR protein of Nostoc punctiforme responsible for transcriptional regulation of genes involved in the differentiation of hormogonia. Mol Microbiol 47: 573–582 [DOI] [PubMed] [Google Scholar]
- Chávez Montes RA, Ranocha P, Martinez Y, Minic Z, Jouanin L, Marquis M, Saulnier L, Fulton LM, Cobbett CS, Bitton F, et al. (2008) Cell wall modifications in Arabidopsis plants with altered α-L-arabinofuranosidase activity. Plant Physiol 147: 63–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu WL, Peters GA, Levieille G, Still PC, Cousins S, Osborne B, Elhai J. (2005) Nitrogen deprivation stimulates symbiotic gland development in Gunnera manicata. Plant Physiol 139: 224–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochran B, Lunday D, Miskevich F. (2008) Kinetic analysis of amylase using quantitative Benedict’s and iodine starch reagents. J Chem Educ 85: 401–403 [Google Scholar]
- Colebatch G, Desbrosses G, Ott T, Krusell L, Montanari O, Kloska S, Kopka J, Udvardi MK. (2004) Global changes in transcription orchestrate metabolic differentiation during symbiotic nitrogen fixation in Lotus japonicus. Plant J 39: 487–512 [DOI] [PubMed] [Google Scholar]
- Cooper JE. (2007) Early interactions between legumes and rhizobia: disclosing complexity in a molecular dialogue. J Appl Microbiol 103: 1355–1365 [DOI] [PubMed] [Google Scholar]
- Ekman M, Tollbäck P, Klint J, Bergman B. (2006) Protein expression profiles in an endosymbiotic cyanobacterium revealed by a proteomic approach. Mol Plant Microbe Interact 19: 1251–1261 [DOI] [PubMed] [Google Scholar]
- Expósito-Rodríguez M, Borges AA, Borges-Pérez A, Pérez JA. (2008) Selection of internal control genes for quantitative real-time RT-PCR studies during tomato development process. BMC Plant Biol 8: 131–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flemetakis E, Efrose RC, Ott T, Stedel C, Aivalakis G, Udvardi MK, Katinakis P. (2006) Spatial and temporal organization of sucrose metabolism in Lotus japonicus nitrogen-fixing nodules suggests a role for the elusive alkaline/neutral invertase. Plant Mol Biol 62: 53–69 [DOI] [PubMed] [Google Scholar]
- Franche C, Lindström K, Elmerich C. (2009) Nitrogen-fixing bacteria associated with leguminous and non-leguminous plants. Plant Soil 321: 35–59 [Google Scholar]
- Hermans C, Hammond JP, White PJ, Verbruggen N. (2006) How do plants respond to nutrient shortage by biomass allocation? Trends Plant Sci 11: 610–617 [DOI] [PubMed] [Google Scholar]
- Kistner C, Parniske M. (2002) Evolution of signal transduction in intracellular symbiosis. Trends Plant Sci 7: 511–518 [DOI] [PubMed] [Google Scholar]
- Knee EM, Gong FC, Gao M, Teplitski M, Jones AR, Foxworthy A, Mort AJ, Bauer WD. (2001) Root mucilage from pea and its utilization by rhizosphere bacteria as a sole carbon source. Mol Plant Microbe Interact 14: 775–784 [DOI] [PubMed] [Google Scholar]
- Kötting O, Santelia D, Edner C, Eicke S, Marthaler T, Gentry MS, Comparot-Moss S, Chen J, Smith AM, Steup M, et al. (2009) STARCH-EXCESS4 is a laforin-like phosphoglucan phosphatase required for starch degradation in Arabidopsis thaliana. Plant Cell 21: 334–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man HM, Silvester WB. (1994) Interactions of H2 and carbon metabolism in moderating nitrogenase activity of Gunnera/Nostoc symbiosis. Arch Microbiol 161: 442–444 [Google Scholar]
- Meeks JC, Elhai J. (2002) Regulation of cellular differentiation in filamentous cyanobacteria in free-living and plant-associated symbiotic growth states. Microbiol Mol Biol Rev 66: 94–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson M, Rasmussen U, Bergman B. (2006) Cyanobacterial chemotaxis to extracts of host and nonhost plants. FEMS Microbiol Ecol 55: 382–390 [DOI] [PubMed] [Google Scholar]
- Rai AN, Soderback E, Bergman B. (2000) Cyanobacterium-plant symbioses. New Phytol 147: 449–481 [DOI] [PubMed] [Google Scholar]
- Rasmussen U, Johansson C, Renglin A, Petersson C, Bergman B. (1994) Early communication in the Gunnera-Nostoc symbiosis: plant-induced cell differentiation and protein synthesis in the cyanobaterium. Mol Plant Microbe Interact 7: 696–702 [Google Scholar]
- Rasmussen U, Johansson C, Renglin A, Petersson C, Bergman B. (1996) A molecular characterization of the Gunnera-Nostoc symbiosis: comparison with Rhizobium- and Argobacterium-plant interactions. New Phytol 133: 391–398 [Google Scholar]
- Seifert GJ, Roberts K. (2007) The biology of arabinogalactan proteins. Annu Rev Plant Biol 58: 137–161 [DOI] [PubMed] [Google Scholar]
- Steinberg NA, Meeks JC. (1991) Physiological sources of reductant for nitrogen fixation activity in Nostoc sp. strain UCD 7801 in symbiotic association with Anthoceros punctatus. J Bacteriol 173: 7324–7329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesfaye M, Samac DA, Vance CP. (2006) Insights into symbiotic nitrogen fixation in Medicago truncatula. Mol Plant Microbe Interact 19: 330–341 [DOI] [PubMed] [Google Scholar]
- Towata EM. (1985) Morphometric and cytochemical ultrastructural analysis of the Gunnera kaalensis/Nostoc symbiosis. Bot Gaz 146: 293–301 [Google Scholar]
- Wang CM, Ekman M, Bergman B. (2004) Expression of cyanobacterial genes involved in heterocyst differentiation and dinitrogen fixation along a plant symbiosis development profile. Mol Plant Microbe Interact 17: 436–443 [DOI] [PubMed] [Google Scholar]
- Wouters J, Janson S, Bergman B. (2000) The effect of exogenous carbohydrates on nitrogen fixation and hetR expression in Nostoc PCC9229 forming symbiosis with Gunnera. Symbiosis 28: 63–76 [Google Scholar]