Abstract
The ability to probe diseases at the genomic level has improved our understanding and enhanced the treatment of breast cancer. One important finding relates to the HER2 oncogene which encodes a novel transmembrane receptor that, when overexpressed, appears to confer growth and survival advantages to breast tumor cells. This fortuitous discovery enabled researchers to develop agents which could inhibit receptor-mediated tumor cell signaling. Numerous clinical trials of such agents have demonstrated improved outcomes in patients with HER2-positive breast cancer. Nonetheless, not all tumors respond to therapy targeting the receptor, while relapses occur after an initial response to treatment. This paper provides a historical and current perspective of the treatment of patients with HER2-positive breast cancer.
Keywords: adjuvant therapy, ErbB, HER2, lapatinib, metastatic breast cancer, neo-adjuvant therapy
HER introduction
Creation and completion of ground breaking clinical trials have led to improved outcomes in patients with breast cancer, especially those with early-stage disease. Supported by compelling evidence collected over the past 40 years,1–4 optimal management of patients with primary operable breast cancer is based on a paradigm of minimal, rather than maximal, therapeutic intervention. Surgical lumpectomy, for example, obviates, or at least attenuates, some of the anatomical and psychological issues associated with mastectomy;1 sentinel node biopsy may circumvent the need for, and preserves arm function better than, complete (axillary) nodal dissection;5,6 and endocrine therapy alone improves survival in patients with early, hormone-responsive breast cancers.7 The latter intervention also embraces the concept that treatment may depend, in part, on identification of unique tumor characteristics. Hence, the ability to probe the disease at the molecular level not only improved our understanding of how estrogens mediated malignant tumor growth but also enhanced our knowledge base, upon which the fertile idea of the estrogen receptor (ER) as a tumor target was conceived.8 A second tumor target surfaced with identification of a novel oncogene that encodes the human epidermal growth factor receptor 2 (HER2) protein.
Even though the discoveries of the ER and HER2 are separated by 2 decades, the receptors appear to be linked in a number of ways. First, both are important breast cancer targets. In fact, the lessons learned with the selective estrogen receptor modulator tamoxifen have been wisely applied to the development of trastuzumab, a humanized monoclonal antibody that recognizes and binds HER2; and while not always appreciated, the concept of “targeted therapy” in oncology really began with tamoxifen. Second, both receptors are predictive markers in that high-level expression of ER and HER2 is associated with (though not absolute) response to therapies directed against their respective targets. The 2 receptors are also prognostic factors; independent of treatment, expression of ER (ie, ER-positive tumors) and absence of HER2 (ie, HER2-negative tumors, except triple negative) correlates with a relatively good prognosis for patients with early breast cancer. Third, and perhaps the most intriguing relationship, is the cross-talk that allegedly occurs between the 2 receptors, a form of communication which may contribute to the development of some tamoxifen-resistant tumor cells.9,10 Also interesting is the finding that despite disease progression, the receptors appear to remain viable targets, which suggests that (at least for a subset of patients) signaling through the receptors continue to mediate tumor cell growth and survival.11–13 Nevertheless, the uncertainty of the mechanisms by which tumors become resistant has negative implications, especially for developing new agents against endocrine- or HER2-refractory disease.
The goal of this paper is to provide an insight on the role and impact of HER2 in breast cancer. As such, events culminating with the discovery of the receptor and development of agents targeting the receptor are reconstructed; clinical trial results leading to drug approval are reviewed; safety data that may soften the benefit to risk ratio are readdressed; and the mechanisms and implications of drug-resistance are reassessed. In order to enhance reader appreciation of the complex processes underlying HER2-mediated breast tumor growth, a brief description of the receptor is discussed initially.
HER family
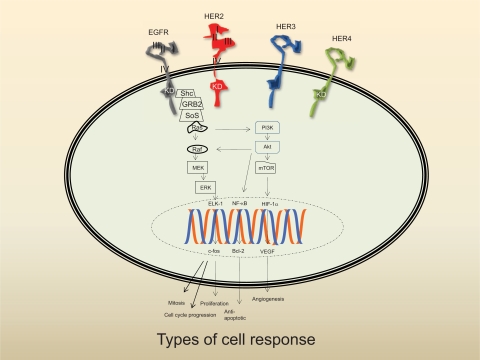
Originally designated as “neu” because of its association with rat neuroblastoma cell lines, the oncogene was believed to be related to an oncogene found in avian erythroblastosis viruses (v-ErbB) that encodes epidermal growth factor receptor (EGFR).14 The substantial homology between EGFR and the neu oncoprotein was also the basis from which HER (human EGFR-related) 2 derived its name.15 HER2/neu is a member of the ErbB family of receptor tyrosine kinases, a homologous group that also includes HER1 (EGFR), HER3, and HER4. Structurally, all members have a short transmembrane that connects the extracellular portion to the intracellular catalytic kinase and regulatory domains (Figure 1).16 Ligand binding initiates a sequence of events including receptor dimerization and kinase phosphorylation, which trigger intracellular signal transduction. The ultimate cellular response depends on recruitment and activation of various protein kinases located downstream of the receptor. Signaling, however, is a phenomenally complex process, more of which will be discussed later. In addition, the authors direct readers to excellent reviews of this topic.17–19
Figure 1.
Schema of the HER family signaling pathways. The linear pathway whereby each component merely functions as a relay switch is grossly oversimplified. The ultimate cellular response is dependent on a diverse array of signals, which is mediated by receptor cross-talk, feedback loops, and counter-regulatory activities. Following ligand binding, the receptor dimerizes and is then phosphorylated. The Ras pathway is especially complex as the kinase must undergo post-translational modification before it can be activated. Phosphorylated HER2 is linked to the Ras signal transduction pathway by binding to 2 adaptor molecules (Shc) and growth-factor receptor bound protein 2 (GRB2). GRB2 forms a complex with son of sevenless (SoS) which is recruited to the plasma membrane leading to activation of Ras. Extracellular signals are propagated from cell surface to nucleus through dynamic interactions with components located downstream of the receptor including PI3K, Akt, and nuclear factor (NF-κβ). Akt blocks apoptosis by antagonizing a proapoptotic member of the Bcl-2 family, Bad, or upregulating Bcl-XL, an anti-apoptotic member of the Bcl-2 family. Akt can also effect angiogenesis via mTor-induced hypoxia inducible factor-1 alpha (HIF-1α) activation. Other downstream effectors of the Ras signal transduction pathway include soluble Raf, mitogen-activated protein kinase kinase (MEK), ERK, and the transcription factors Elk-1 and c-Fos, a nuclear proto-oncogene that regulates expression of cyclin-dependent kinases (CdK).
Notes: 1) domains I and III are believed to be the primary ligand binding site. The “extended” or closed conformer of HER2 precludes ligand access; 2) the absence of the kinase domain (KD) on HER3 represents the impairment of this receptor; and 3) PI3K preferentially binds to phospho-HER3.
Physiologically, the ErbB family has long been known to contribute to the development of a number of important organs and tissue systems.20,21 Although not restricted to mammary tissue, studies of HER isoforms suggest each receptor has a different function in normal gland development. While HER2 promotes lobuloalveolar differentiation and lactation, HER1 contributes to ductal growth.22 Paradoxically, compelling evidence linking these receptors to human neoplasia, including breast cancer, had been reported over 2 decades ago.15,23,24 For instance, high levels of the HER2 and EGFR have been found in breast cancer (as well as a number of other solid tumors).25 In addition, amplification of the HER2-oncogene or overexpression of the oncoprotein has been shown to convey tumor cell growth and survival advantages.26 Although overexpression is paramount to the transformed state, it remains unclear whether HER2 aberrations arise very early (ie, hyperplasia) or much later (ie, neoplasia) in the tumorigenic process. The determination of when this functional abnormality occurs may be crucial as earlier detection may improve the prognosis of a significant number of patients.
HER presence
Amplified (gene) or overexpressed (receptor) in approximately 20% to 25% of breast cancers, HER2-positivity is associated with a number of adverse tumor characteristics involving size, nuclear grade, S-phase fraction, and ploidy.27 Perhaps more important is the clinical correlation between receptor-positive tumors and higher risks of relapse, shorter time to disease progression, and poorer overall survival.28 The significance of the genomic abnormality was important because positive correlation between HER2 gene amplification and breast cancer formed the basis for the development of a useful clinical test29,30 as well as an effective therapeutic strategy.31
It is conceivable that amplification and/or overexpression of the three other HER-related family members may also influence the clinical course and outcomes of HER2-positive breast cancer. For example, data linking co-expressed EGFR with increased HER2 signaling activity and poorer overall prognosis have been published.32,33 There is also striking evidence for the negative impact of altered HER3 on disease outcome in patients with breast cancer.34 Furthermore, others have reported that any combination of EGFR, HER2, and HER3 was associated with significantly reduced disease-specific survival.35 In contrast, expression of HER4 has frequently been related to a more favorable breast cancer prognosis.36 Because of their retrospective nature, the clinical significance of the co-expression data is still uncertain. This conclusion is supported by two findings. First, while information related to HER2 overexpression is usually remarkably similar (ie, 23% to 27%), analysis of EGFR (16% to 36%), HER3 (18% to 26%), and HER4 (12% to 82%) overexpression indicates more variabilty.36–38 Second, expression of these receptors may, interestingly, increase or decrease during the course of the disease.39 Nonetheless, prospective quantification of these receptors may provide clinically relevant information related to disease outcome.
HER therapy
Even though only two agents (that target the receptor) have received FDA approval for use in patients with HER2-positive breast cancer, their impact, especially trastuzumab (Herceptin™; Genentech, Inc.), have been substantial. Several phase III and II clinical trials (discussed later) with lapatinib (Tykerb™; GlaxoSmithKline) suggest that the newer agent is at least as active as trastuzumab. What may also be learned about these 2 agents is whether attenuation of receptor signaling (and hence, antitumor effect and cross-resistance) differs with respect to the external or internal drug-binding sites.
Trastuzumab
Trastuzumab is a recombinant humanized monoclonal antibody that recognizes and binds with high affinity to an epitope on the extracellular domain of HER2. Notably, trastuzumab does not “compete” with an endogenous ligand for the receptor binding site because none has, as yet, been identified. Even though antibody-mediated blockade of receptor activation is believed to contribute to the antitumor effect, this is a gross oversimplification of the mechanisms (which remain ill-defined) by which trastuzumab induces tumor regression. Nonetheless, 2 mechanistic hypotheses have been proffered. The first involves perturbation of a number of signal transduction pathways following receptor endocytosis and proteolysis. As such, signaling through key molecules located “downstream” of the receptor, such as phosphatidylinositol 3 kinase (PI3K) and mitogen-activated protein kinase (MAPK), is disrupted.40 Furthermore, since ErbB receptors can be recycled to the cell membrane in a functional state, it is believed that trastuzumab enhances lysosomal breakdown of HER2 by recruiting a protein known as c-Cbl.41 This theory, however, has been challenged on the basis that surface downregulation is not an important mechanism of drug action.42 Tumor cells treated with trastuzumab also exhibit growth arrest in G1 phase which is due, in part, to decreased amounts of proteins involved in the separation of p27kip1 and the cyclin E/cdk2 complex.43,44 Interestingly, it has also been reported that the antibody blocks angiogenesis by modulating the release of proangiogenic and antiangiogenic factors.45,46 Two other mechanisms that may contribute to antitumor activity include the ability to block DNA repair, an effect partially mediated by modulating p21WAF1 and formation of highly active truncated HER2 by inhibiting proteolytic cleavage of the extracellular domain (ECD).47,48 While downregulation of HER2 is a plausible mechanism, several studies provide conflicting results suggesting that trastuzumab does not alter any of the previous findings, even the ability to cause apoptosis as the terminal event.42,49,50
An alternative, though not necessarily mutually exclusive, explanation of the function of trastuzumab involves the recruitment of an immune component. Strong evidence suggests that the antibody binds and activates Fc receptors expressed on immunocompetent lymphocytes and NK cells resulting in antibody-dependent cellular cytotoxicity (ADCC).51 Although persuasive, the phenomenon of ADCC may not be important, as inhibition of tumor growth has been observed with antibodies that lack the Fc fragment.52
Trastuzumab can be given in 2 different schedules. While most investigators used a 4 mg/kg loading dose followed by once weekly doses of 2 mg/kg, the results of other studies suggest an equally effective and more “patient-friendly” schedule of 6 mg/kg given every 3 weeks following an 8 mg/kg loading dose. The latter schedule, based on the drug’s estimated half-life which ranges from 18 to 27 days, has also been shown to achieve steady-state blood concentrations (of 50–60 μg/mL), which is above the effective level of approximately 10 to 20 μg/mL.53
Lapatinib
Lapatinib, a small molecule that functions as a competitive inhibitor of both EGFR and HER2 tyrosine kinases, has been shown to cause prolonged downregulation of tyrosine phosphorylation in tumor cells.54 Because receptor phosphorylation induces recruitment and activation of numerous downstream effectors which ultimately control cell destiny, disruption of receptor-mediated signals can affect cell survival. One of the most important intermediaries affected is members of the Ras gene family (Figure 1). The Ras proteins are small guanosine triphosphate (GTP)-binding proteins that play principal roles in regulating cell proliferation.55 Ras propagates signals emanating from the cell surface to the nucleus through dynamic interactions with other effector components. For example, binding of PI3K to Ras activates Akt, which enhances cell survival and stimulates new blood vessel formation.56,57 A number of other signaling effectors downstream of Ras include Raf gene family members, MAPK, ERK (extracellular signal-regulated kinase), and the transcription factors Elk-1 and c-Fos.58 However, because lapatinib’s inhibitory effect occurs at the level of the receptor, gene mutations or protein abnormalities of any of the intermediaries distal to the receptor are likely to confer resistance to lapatinib.
Lapatinib is administered orally at a dosage of 1250 mg daily.
Treatment-related toxicities
Safety data from clinical trials indicate that cardiac failure or decreases in left ventricular ejection fraction (LVEF) are the major toxicities associated with trastuzumab therapy.59,60 Although the precise mechanism is not known, it has been postulated that contractile dysfunction may result from blocking a HER2 pathway that mediates growth and survival of cardiac smooth muscle cells.61–63 The most notable finding emanated from the trastuzumab licensing trial involving patients with metastatic breast cancer.31 In this study, patients treated with first-line trastuzumab plus an anthracycline had a 16% risk of developing class III/IV (New York Heart Association) cardiotoxicity. Another study of patients (the majority of whom were exposed to anthracyclines) with advanced breast cancer treated with trastuzumab for a median of nearly two years reported an 11% incidence of grade III cardiac events.64 Symptomatic or LVEF improvement occurred in nearly all patients following discontinuation of the antibody and institution of pharmacologic therapy for cardiac dysfunction. One of the major clinical anomalies is that the relatively high incidence of serious cardiac toxicity in these 2 studies has not been observed in other clinical trials of combined treatment with trastuzumab and an anthracycline.65–67
When used as monotherapy for metastatic breast cancer, cardiac toxicity develops in approximately 4.5% of patients who received prior anthracycline therapy.68 Cardiotoxic reactions, however, do not appear to be increased only with anthracyclines as these events have also been observed in studies of trastuzumab and paclitaxel.69–71 Although the incidence is approximately 4% when the two agents are given concurrently, the occurrence of cardiac toxicity is <1% with sequential administration of trastuzumab (ie, following completion of paclitaxel therapy). Collectively, these data suggest that: 1) the development of high-grade cardiac toxicity is more closely associated with the chemotherapeutic agents in the regimen71–73 (the seriousness of this toxic event has led to the conclusion that trastuzumab should not be given with anthracycline-containing regimens)74 and the schedule of trastuzumab administration; and 2) the myocardial damage can be partly attributed to the antibody itself. Nevertheless, outcomes data strongly suggest that combining trastuzumab with or following chemotherapy in the adjuvant setting results in benefits unparalleled by most available therapies for solid tumors. Addition of trastuzumab to chemotherapy should be strongly considered for patients with HER2–positive early-stage breast cancer, especially those at low risk for cardiovascular morbidity.
Interestingly, an extensive review of cardiac function in a large cohort of patients treated with lapatinib either as monotherapy or in combination with cytotoxic agents indicates that lapatinib does not appear to confer a high risk of developing heart failure.70 Of the 3558 patients, 58 (1.6%) had confirmed decreases in LVEF. Furthermore, only 7 of the 58 patients (0.2%) were symptomatic. In addition, nearly all of the patients with decreased LVEF had medical or prior treatment histories that could have contributed to the cardiac event. Hence, even though signaling through HER2 is disrupted, lapatinib may be less cardiotoxic than trastuzumab. Part of the explanation may include the finding that lapatinib induces adenosine monophosphate kinase, which plays a key role in protecting cells against ischemic damage.75
HER trials
Results of early studies demonstrated that monotherapy trastuzumab was effective in patients with advanced breast cancer either as first-line therapy or in patients with disease progressing after chemotherapy.76,77 Because of the relatively low response rates (ie, <25%) observed in patients participating in these studies, as well as preclinical evidence suggesting that the antibody may enhance the antitumor effect of chemotherapy,78 a number of clinical trials were performed to determine whether combining the two types of therapies were better than either alone. The most important data were obtained in a pivotal phase III trial which showed that addition of trastuzumab to chemotherapy (doxorubicin plus cyclophosphamide or paclitaxel) resulted in superior outcomes compared to chemotherapy alone in all clinical endpoints (Table 1).31 In this study, women with HER2 overexpressing metastatic breast cancer who were randomized to trastuzumab plus chemotherapy achieved statistically significant improvement in overall response rates (ORR, 50% vs 32%; P < 0.001), time to tumor progression (TTP, 9.1 vs 6.1 months; P < 0.001), and overall survival (OS, 25.1 mo vs 20.3 mo; P < 0.05). Importantly, more than half of the patients treated with chemotherapy alone received the antibody at the time of disease progression. Additional data confirming the efficacy of the paclitaxel-containing doublet emanated from a phase II trial, which compared weekly administration of the taxane with or without trastuzumab.79 Patients with HER2-positive tumors (scored as 2+ or 3+ by immunohistochemistry [IHC]) who received the combination, achieved significantly better ORR (84.5% vs 47.5%; P = 0.0005) and median TTP (369 days vs 272 days; P = 0.03). Both treatment arms were well tolerated; no cardiac events or grade 4 myelosuppression were observed. A consistent finding in both of these studies was the superior outcomes among patients with IHC 3+ tumors.
Table 1.
Selected clinical trials of trastuzumab in breast cancer
| Design | Key eligibility criteria | Treatment schema | Endpoints | Outcomes |
|---|---|---|---|---|
| Advanced disease | ||||
| Phase III, randomized; 469 patients enrolled31 | HER2-overexpressing (IHC 2+ or 3+), previously untreated, metastatic breast cancer | No prior anthracycline Doxorubicin (A) 60 mg/m2 (or epirubicin 75 mg/m2) plus cyclophosphamide (C) 600 mg/m2 q 3 wk × 6 cycles ± trastuzumab (H) 4 mg/kg × 1, then 2 mg/kg weekly till disease progression Prior anthracycline Paclitaxel (T) 175 mg/m2 q 3 wk × 6 cycles ± trastuzumab as above |
10 – TDP and incidence of adverse effects 20 – ORR, DOR,TTF, and OS |
Data below are median values in patients treated with chemotherapy + trastuzumab vs chemotherapy alone: TDP (7.4 mo vs 4.6 mo; P < 0.001) ORR (50% vs 32%; P < 0.001) DOR (9.1 mo vs 6.1 mo; P < 0.001) TTF (6.9 mo vs 4.5 mo; P < 0.001) OS (25.1 mo vs 20.3 mo; P = 0.046); calculation includes patients who received trastuzmab after disease progression on chemotherapy alone Patients with 3 + HER2 scores had greater benefit than 2 + tumors |
| Adverse events associated with trastuzumab therapy: All cardiac toxic events: AC + H = 27% (39 of 143 patients) AC alone = 8% (11 of 135 patients) T + H = 13% (12 of 91 patients) T alone = 1% (1 of 95 patients) | ||||
| Chills and/or fever (25%) Infections (47% compared to 29% of patients treated with chemotherapy alone) | ||||
| Phase II open-label, randomized, efficacy and safety as first-line therapy; 186 patients enrolled82 | HER2-overexpressing (3+ IHC or FISH-positive) metastatic breast cancer | Docetaxel (D) 100 mg/m2 ± H same dose/schedule indicated above | 10 – ORR 20 – TDP,TTF, DOR, OS, and safety profile |
Data below are median values in patients treated with D + H vs D alone: ORR (61% [6 CRs, 7% and 50 PRs 54%] vs 34% [2 CRs, 2% and 30 PRs, 32%]; P = 0.0002) TDP (11.7 mo vs 6.1 mo; P = 0.0001) TTF (9.8 mo vs 5.3 mo; P = 0.0001) DOR (11.7 mo vs 5.7 mo; P = 0.009) OS (31.2 mo vs 22.7 mo; P = 0.0325) |
| Adverse events associated with trastuzumab therapy: Grade 3–4 neutropenia (32% vs 22%) Febrile neutropenia (23% vs 17%) Symptomatic cardiac event (1 patient) | ||||
| Adjuvant therapy | ||||
| HERA phase III open-label, randomized; 3388 evaluable patients84 | Patients with HER2-overexpressing (3+ IHC or FISH-positive) breast cancer and completion loco-regional and systemic neo-adjuvant and adjuvant therapy. All patients required to have normal LVEF | Randomization to:
|
10 – DFS 20 – site of first DFS event, TDR, OS, and cardiac safety |
Efficacy analysis, based on intention-to-treat principle, reported below compares patients assigned to 1 yr H vs control: DFS events (127 vs 220; P < 0.0001) TDR (HR 0.49; P < 0.0001) OS (96% vs 95.1%; P = 0.26) |
| Cardiac events: Class III/IV heart failure (9 patients vs 0; P = 0.002) Symptomatic CHF including class III/IV (29 patients vs 1 patient; P < 0.001) Decrease in LVEF to < 50% (113 patients vs 34 patients; P < 0.001) | ||||
| NSABP (B-31) and NCCTG (N9831) phase III open-label, randomized; 3351 evaluable patients69 | Patients with HER2-overexpressing (3+ IHC or FISH-positive) and node-positive or high-risk node-negative breast cancer. All patients required to have normal LVEF | B-31 randomization:
|
10 – DFS 20 – TDR, OS, and death due to breast cancer or other second malignancy |
For the analysis, combined data from groups 1 and A were compared to data from groups 2 and C; median follow-up was 2 yr DFS events (261 vs 133; P < 0.0001) TDR (HR 0.47; P < 0.0001) OS (94.3% vs 91.7%; P = 0.015) Death from breast cancer (79 patients vs 53 patients; P = 0.02) Death due to second primary (20 patients vs 5 patients; P = 0.002) Class III/IV CHF, 3 yr cumulative incidence (0% vs 2.9% of which one patient died of cardiac toxicity) |
N9831 randomization:
| ||||
| FinHer Phase III randomized, open-label; 1010 patients enrolled86 | Patients, status post breast surgery, node-positive or high-risk node-negative, confirmed HER2 amplification by chromogenic in-situ hybridization | Randomization (4 arms): D at 100 mg/m2 q 3 wk × 3 cycles ± 9 total doses of H at 4 mg/kg beginning with first dose of D, then 2 mg/kg weekly × 8 followed by 3 cycles of fluorouracil (F) 600 mg/m2, epirubicin (E) 60 mg/m2, cyclophosphamide (C) 600 mg/m2 q 3 wk | 10 – RFS 20 OS and cardio toxic events |
Analyses were limited to 232 (of 1010) patients who had an amplified HER2 gene; of the 232 women, 116 who received H are compared to 116 who did not RFS at 3 yr (12 [10.7%] vs 27 [22.4%] patients with recurrence; P = 0.01) OS at 3 yr (6 [5.2%] vs 14 [12%] patients died; P = 0.07) |
| Vinorelbine (V) 25 mg/m2 days 1, 8, and 15 q 3 wk × 3 cycles ± H at same dose/schedule listed above followed by FEC as noted above | Cardiac events (3 LVEF < 50% and 1 myocardial infarction; none received H) RFS at 3 yr (HER2+ treated with H vs HER2–, no H; P = 0.82) | |||
| BCIRG 006 phase III randomized trial; 3222 evaluable patients92 | Patients with HER2-overexpressing (FISH-positive) and node-positive or high-risk node-negative breast cancer | Randomization:
|
10 – DFS 20 – OS and symptomatic cardiac events |
2nd interim analysis of patients randomized to groups 1 (n = 1073), 2 (n = 1074), and 3 (n = 1075) with a median follow-up of 3 years; comparisons were made between H-containing regimens and group 1: DFS (88.1% [ACDH] and 86.8% [DCH] vs 82.1%; P < 0.0001 and P = 0.0003, respectively) OS (95.4% [ACDH] and 94.8% [DCH] vs 92.5%; P = 0.004 and P = 0.017, respectively) DFS and OS (no difference between H-containing groups) |
| Symptomatic cardiac events (0.4% [ACD and DCH] vs 1.9% [ACDH]) | ||||
| Neoadjuvant therapy | ||||
| NOAH Phase III randomized, open-label, evaluating addition of H to standard pre-operative chemotherapy; enrolled 228 patients89 | Patients with locally advanced HER2-positive breast cancer (T3N1 or any T plus N2 or N3 or ipsilateral supraclavicular node involvement) | Treatment randomization prior to surgery:
|
10 – EFS 20 – cPR, ORR, and OS |
Analysis compares H-containing regimen and chemotherapy alone; at a median follow-up of 3 years: EFS (70% vs 53%; P = 0.006) ORR (89% vs 77%; P = 0.02) PCR (39% vs 20%; P = 0.002) OS (not significant) Cardiac toxicity (95% of patients had common toxicity criteria values of 0–1) |
| Phase III prospective, randomized; target enrollment of 164 patients87 | Patients with stage II or IIIA locally advanced, noninflammatory HER2-positive (FISH-positive or 3+ IHC) breast cancer | Treatment randomization prior to surgery:
|
10 – 20% improvement in pCR |
Trial stopped after 34 patients had completed therapy because of superiority of the H-containing regimen pCR (66.7% vs 25%; P = 0.02). Cardiac events (no clinical CHF observed) |
| Phase II open-label trial; 33 patients enrolled88 | Patients with stage II or III locally advanced, non-inflammatory HER2-positive (3+ IHC) breast cancer | Treatment prior to surgery:
|
10 – pCR 20 – OR (CR and PR), BCS, DFS, local and distant relapse, and safety |
Intention-to-treat analysis confined to 29 patients who completed therapy pCR (tumor and node, 47% of patients) OR (CR, 73%; PR 23%) BCS (23 [77%] patients) Grade 3–4 neutropenia (85% of patients) Febrile neutropenia (18% of patients) No cardiotoxic events |
Abbreviations: BCS, breast-conserving surgery; CHF, coronary heart failure; cPR, complete pathologic response; EFS, event-free survival; HER2, human epidermal growth factor receptor 2; IHC, histochemistry; LVEF, left ventricular ejection fraction; NSABP, National Surgical Adjuvant Breast and Bowel Project; ORR, overall response rate; DOR, duration of response; TTF, time to treatment failure; ORR, overall response rate; OS, overall survival; RFS, recurrence-free survival; TDP, time to disease progression; TDR, time to distant recurrence.
Because of the reported synergy between trastuzumab and docetaxel in preclinical models, a phase II trial evaluated the combination of the 2 agents in patients with HER-2 overexpressing metastatic breast cancer.80 Thirty subjects were treated with weekly doses of docetaxel and trastuzumab. One novel finding related to the presence, and correlation, of the extracellular domain (ECD) (of HER2) with response rate (RR). In patients with elevated HER2 serum ECD at baseline the RR was 76%; patients with low serum ECD levels had a RR of 33%.81 These findings were consistent with another study of trastuzumab plus docetaxel as first-line treatment of HER2-positive metastatic breast cancer (Table 1).82 Compared to docetaxel alone, the combination was significantly more effective in all tumor endpoints including ORR (61% vs 34%; P = 0.0002), median OS (31.2 vs 22.7 months; P = 0.0325), median time to disease progression (11.7 vs 6.1 months; P = 0.0001), median time to treatment failure (9.8 vs 5.3 months; P = 0.0001), and median duration of response 11.7 vs 5.7 months; P = 0.009). Again, superior clinical outcomes correlated with tumors bearing gene-amplification and/or oncoprotein overexpression. The most notable toxicities observed in patients who received both agents were the higher incidence of neutropenia and neutropenic fever and the death of 1 patient due to heart failure.
A phase III trial of paclitaxel with or without lapatinib, as first-line treatment for metastatic breast cancer has also been conducted.83 Interestingly, patients enrolled (n = 579) had tumors that were either HER2-negative or unknown at the time of study entry. Consistent with previous findings, no differences were observed in all endpoints regardless of treatment. However, among the 86 patients with HER2-positive breast cancer, treatment with the combination resulted in significant improvements in TTP, event-free survival, ORR, and clinical benefit rate.
Having demonstrated that patients with advanced disease obtained significant benefit with trastuzumab, it was proposed that those with early stage HER2-positive breast cancer might also achieve substantial improvement in clinical outcomes. To address this hypothesis, 5 clinical trials were conducted to determine the relative importance of adding trastuzumab to standard adjuvant chemotherapy (Table 1).69,84,86,92 The HERA (Herceptin Adjuvant) Trial investigated the efficacy of trastuzumab (compared to observation only) when given after completion of loco-regional therapy and at least 4 cycles of adjuvant chemotherapy for 1 or 2 years.84 Trastuzumab was administered every 3 weeks. The primary endpoint of the trial was disease-free survival (DFS), which was defined as any event including breast cancer recurrence, new second primary malignancy, or death. Thus far, analysis of only patients (n = 1694) randomized to the 1-year treatment arm (compared to control, n = 1693) has been reported. The initial publication (after a median follow-up of 1 year) indicated that DFS was significantly superior in patients who received trastuzumab (85.8% vs 77.4; P < 0.0001). The absolute benefit of 8.4% corresponded to a 46% lower risk of developing an “event” (unadjusted hazard ratio [HR] of 0.54; 95% CI 0.43–0.67). This degree of benefit in early breast cancer represents the largest to be reported since the introduction of tamoxifen. At 2 years median follow-up, 59 and 90 patients have died in the trastuzumab and control groups, respectively. Analysis of these data showed a 34% reduction in risk of death with trastuzumab (HR 0.66, 95% CI 0.47–0.91; P = 0.0115) compared to the observation alone arm indicating a significant overall survival benefit.85 Similarly, the HR for the risk of a DFS event was 0.64 (95% CI 0.54–0.76; P < 0.0001).
Two other phase III trials compared trastuzumab given concurrently with, or following completion of, adjuvant chemotherapy in women with surgically resectable, HER2-postive (ie, 3+ by IHC or gene amplification detected by fluorescent in-situ hybridization [FISH]) breast cancer (Table 1). The combined results were reported in one publication.69 One, conducted by the National Surgical Adjuvant Breast and Bowel Project (NSABP B-31 trial), compared four 21-day cycles of doxorubicin and cyclophosphamide (AC) followed by four 21-day cycles of paclitaxel (T) (AC→T) to the same regimen plus 52 weekly doses of trastuzumab (H) beginning with the first paclitaxel dose (AC→TH); the other was North Central Cancer Treatment Group trial (NCCTG) N9831, which compared the same agents and same sequence (except T was given weekly for a total of 12 doses) and AC followed by concurrent administration of T plus trastuzumab, which was given weekly for 1 year. Similar to the HERA trial, the primary endpoint was DFS. At a median follow-up period of 2 years, 261 events had occurred in the control arm compared to 133 in the trastuzumab-treated arm (HR 0.48, 95% CI 0.39–0.59; P < 0.0001). The absolute difference in percentage of patients alive and disease-free increased from 11.8% (at 3 years follow-up) to 18.2% (at 4 years follow-up) favoring the group receiving trastuzumab. In addition, the absolute difference in overall survival also favored the trastuzumab group, increasing from 2.6% to 4.8% (P = 0.015) at 3 years and 4 years follow-up, respectively. These highly significant differences crossed the a priori determined early stopping boundary and provided further evidence of the remarkable benefit attributable to trastuzumab.
Based on the positive outcomes as adjuvant therapy, it was hypothesized that pre-operative administration of trastuzumab plus chemotherapy could be beneficial in patients with HER-2 positive, locally advanced or inflammatory breast cancers. As such, neo-adjuvant therapy has the potential not only to render inoperable tumors resectable, but also lower rates of recurrence and cancer-related deaths in a group of patients who are almost certain to do poorly. Over the past 4 years, several groups have reported significantly higher pathologic complete responses (pCRs), an important predictor of superior DFS and OS in small numbers of patients who received trastuzumab as part of the treatment regimen (Table 1).87,88 These early findings were supported by results from a large phase III clinical trial that was presented recently.89 Compared to chemotherapy alone, patients who received chemotherapy plus trastuzumab have significantly higher pCR rates (20% vs 39%; P = 0.002) and event-free survival rates, 53% vs 70% (HR, 0.56; P = 0.006). Furthermore, in contrast to 1 of the early studies,86 the improved outcomes in this trial was observed in patients regardless of concomitant ER status.
The clinical activity and safety of lapatinib has also been investigated in patients with HER2-overexpressing or EGFR-positive advanced inflammatory breast cancers.90 Objective responses were observed in 15 of the 30 women with HER2-positive disease; only 1 of the 15 patients with EGFR-positive/HER2-negative tumors achieved a clinical response. Notably, prior trastuzumab therapy did not influence response to lapatinib. Although not currently approved for use in this setting, it is likely that trastuzumab will receive FDA approval as part of neo-adjuvant therapy in the near future.
Nonetheless, the concern for cardiac toxicity in patients receiving trastuzumab plus chemotherapy (especially anthracyclines) has pushed investigators to find alternative treatment regimens with equal efficacy and better tolerability. Based on promising preclinical data combining carboplatin with paclitaxel, a phase III study was performed to evaluate the efficacy and safety of trastuzumab plus paclitaxel with our without carboplatin.91 As front-line therapy for advanced breast cancer, patients randomized to the 3-drug regimen had significantly better ORR (52% vs 36%; P = 0.04) and median progression-free survival (10.7 vs 7.1 months; P = 0.03). The only serious toxicity that occurred more frequently with the triple drug regimen was grade 4 neutropenia.
While the taxanes and anthracyclines are among the most effective agents used in the treatment of breast cancer, combinations of trastuzumab with other agents such as vinorelbine,93 gemcitabine,94 and capecitabine95 have also been reported to be quite active.
Despite clinical confirmation that HER2 is an important tumor target and that selective targeting can be achieved, there are several realities that must be emphasized. First, in spite of early benefit, tumor progression occurs in virtually all patients with advanced disease and in a significant number of patients with early breast cancer; 31,69,84 second, only modestly active as single agents, tumor outcomes are significantly improved when either agent is combined with chemotherapy; and third, although relatively well tolerated, cardiotoxicity is associated with HER2-targeted agents.72
Based on the results of phase III clinical trial, lapatinib provides some measure of hope for patients with tumors progressing on trastuzumab and chemotherapy.72 Originally designed to include 528 women, enrollment was terminated and data locked following an interim analysis of efficacy and safety data by an independent monitoring committee. At that time, 324 patients had been randomized to capecitabine plus lapatinib or capecitabine alone. On the basis of 121 events, 49 in the combination and 72 in the monotherapy groups, a 51% improvement in TTP (8.4 months and 4.4 months; P < 0.001) favored the group receiving capecitabine and lapatinib. A secondary endpoint, progression-free survival, defined as the time from randomization to disease progression or death from any cause, was also improved in the combination-therapy group (HR 0.47; 95% CI 0.33–0.67; P < 0.001). In addition, central nervous system involvement was significantly lower with combination therapy compared to capecitabine alone, 2% versus 11%, respectively, P = 0.0445. The latter finding is of immense interest because the development of brain metastases occurs in approximately one-third of patients with advanced HER2-positive breast cancer. A small phase II study was conducted in women to evaluate the effect of lapatinib on progressive brain metastases despite prior radiation therapy.96 Among 39 subjects who were previously treated with trastuzumab, a PR was achieved in one patient; further progression was not observed in seven other patients 16 weeks after beginning lapatinib.
Further information related to completed and ongoing clinical trials of both agents can be accessed at: http://www.cancer.gov/cancertopics/druginfo/trastuzumab and http://www.cancer.gov/cancertopics/druginfo/lapatinibditosylate.
HER secrets
A number of interesting findings suggest that HER2 may be the most intriguing member of the ErbB family.16,17 Of note, at least 7 EGF-like molecules have been shown to bind EGFR, HER3 and HER4; none, however, binds to HER2. The reason why identification of a stimulating ligand remains elusive could be related to HER2 itself. Analysis of the crystal structure exposed a pair of clarifying facets about the receptor: 1) the extended conformation of monomeric HER2 may hinder binding of any EGF-like peptide (Figure 1);97 and 2) structural defects at the receptor binding site appear to be the result of unconserved or altered amino acid residues.99
While ligand binding triggers receptor dimerization, the absence of specific stimulatory molecules does not preclude kinase activation. Indeed, overexpression of HER2 results in formation of receptor homodimers that are constitutively active.99 Furthermore, a cursory glance at the intracellular signaling cascade could result in the erroneous conclusion that activated HER2 only has a rudimentary role in trafficking signals down a vertical transduction pathway (Figure 1). Signaling, however, is phenomenally diverse and complex, in large part, because HER2 is believed to be the preferred dimeric partner of the other ErbB family members.100 This feature has profound implications. For example, the dimeric partner and its activating ligand play essential roles in determining not only which receptor sites are phosphorylated, but also which downstream proteins are recruited.101 The result is an expansion of the signaling repertoire. Moreover, formation of HER2 heterodimers may be of pathologic importance. This conclusion is supported by the surprising finding that although neither monomeric HER2 (ligand-less) nor HER3 (kinase-impaired) can support linear signaling alone, the combination appears to possess the most potent mitogenic properties.102 Similarly, tumorigenic signals emanating from the EGFR/HER2 heterodimer are stronger compared to either homodimer alone.103 These bi-partisan relationships may also be clinically relevant as the poor prognosis associated with tumors overexpressing HER2 could be partly due to transactivation of the receptor’s intrinsically high tyrosine kinase activity by the consorting partner. Reports of patient tumors continuing to respond to trastuzumab even after initial disease progression104–107 further suggest that part of the HER2 mystique may be related to another member of the HER family. Additional evidence to support this conclusion is provided by preclinical models. Analysis of mice bearing a breast cancer xenograft that co-expresses EGFR and HER2 revealed the formation of EGFR and full-length HER2 (p185ErbB2) heterodimers.108 More important, phosphorylation of 2 key downstream proteins was inhibited only when both receptor kinases were blocked. This finding provides a possible explanation for the efficacy of lapatinib in tumors where trastuzumab had little effect.
Molecularly, the importance of HER2 extends even further as the full-length protein (p185ErbB2) undergoes proteolytic truncation resulting in a deceptively shortened receptor (p95ErbB2) with increased autokinase activity.109 Clinically, elevated serum levels of the dissociated ECD have been correlated with poorer responses to therapy (which contrasts with another report)81 as well as lymph node metastasis.110,111 The presence of cleaved HER2 is notable for 2 other reasons. First, the truncated receptor preferentially dimerizes with HER3,108 and second, phosphorylation of p95ErbB2 can be blocked by lapatinib but not trastuzumab.108 Thus, the heterodimer concept could explain the effectiveness of lapatinib in patients who fail trastuzumab. Intriguingly, the answer may be partly related to EGFR. While formation of antibody–receptor complexes is believed to undergo endocytosis and subsequent proteolytic degradation, specific inhibition of the EGFR tyrosine kinase have been shown, both ex vivo and in vivo, to affect heterodimer formation resulting in marked impairment of HER2 signaling.112–114
The importance of HER2 heterodimers in normal developmental processes also provides a clue to explain one of the major toxicities associated with anti-HER2 therapy. Based on the biological abnormalities of HER2 and HER3, it is now known that a family of EGF-like glycoproteins known as the heregulins (HRG) can overcome the limitations of each receptor. Although HRGs bind to HER3 and HER4, one of the active complexes through which HRG signaling occurs is the HER2/HER3 heterodimer.102 The relevance of this finding has been observed in preclinical models, which demonstrate the critical role of HER2 and HER3 in development and differentiation of cardiac myocytes.115 Knockout of the HRG and HER2 genes resulted in thinning of the ventricular wall and complete absence of myocyte trabeculation, which led to cardiac hypertrophy, dysrhythmias, and vascular rarefaction. Embryonic viability ceased by day 11. The relative importance of HER3 was also demonstrated using HER3−/− (ie, knockout) mouse embryos, which resulted in significant valvular defects. So severely underdeveloped, the valves were unable to support normal cardiac function causing mitral and aortic regurgitation.116 Thus, the pathogenic mechanism of trastuzumab-associated myocardial dysfunction could be partly related to inhibition of signaling through HER2 or blocking HER2-mediated transactivation of HER3.
HER2 may be at the focus of yet another malevolent relationship, one that involves the ER. Although the connection could be a manifestation of genetic evolution, it is more likely to be one that has been genetically conserved since growth factor pathways have been demonstrated to influence, and be influenced by, ERα signaling.117 Compelling evidence suggest that estrogen deprivation strategies are associated with overexpression of heregulin,118 as well as HER1 and HER2, which may contribute to the development of resistance to endocrine therapies in breast cancer.119,120 Preliminary results of an important clinical trial to test this hypothesis have been recently reported.121 Eligibility was based on confirmation of positive hormone receptor disease regardless of HER2 status. Of the nearly 1300 patients enrolled, 219 of them had tumors that were HER2-positive also. In patients with double receptor-positive tumors, addition of lapatinib to letrozole improved the RR (from 15% to 28%) and progression-free survival (from 3 months to 8.2 months). No differences in endpoints were observed between treatment groups in patients with HER2-negative tumors.
Finally, mortality from breast cancer will be even lower if the disease could be prevented or were altogether less common. Three clinical studies, each based on the association between estrogens and breast cancer, have demonstrated that chemoprevention can reduce the risk of developing invasive breast cancer in patients at high-risk for the disease.122–124 Despite the efficacy of tamoxifen and raloxifene, neither agent reduces the occurrence of ER-negative tumors. Tethered to this finding is another possible outcome, one that is truly hypothetical, though within the realm of reality. Because laboratory studies have shown that estrogen deprivation upregulates the HER2 signaling pathway, one concern relates to the possible development of HER2-postive breast cancer due to widespread application of chemopreventive therapy. Part of the answer may indirectly be provided by a phase III trial (EGF30008) involving patients with ER-positive breast cancer who are randomized to receive an aromatase inhibitor with or without a HER2 antagonist.
HER destiny
Despite the voluminous amount of new information that has been published, it is humbling to note that besides being a valid target, only a small portion of the receptor’s complete biology is apparently known. The lack of full understanding underlies our inability to explain why anti-HER2 therapy is not effective in all tumors that overexpress the receptor or why continuation of the antibody is beneficial in some patients who progressed on a trastuzumab-containing regimen. Because cancer investigations have provided convincing evidence that multiple signaling defects exist in most solid tumors, including breast cancer, it would be reasonable to suggest that dual- or multikinase inhibitors will be more efficient at eradicating HER2-positive tumor cells. Still, it remains unclear whether lapatinib (or any other multikinase inhibitor) will be more effective than trastuzumab. It is highly probable that one agent will be active in some tumors that are resistant to another agent. At the same time, there is an opportunity to assess whether combined therapy (ie, targeting HER2 from the “outside” and “inside” simultaneously) is significantly better than either type of agent alone. Preliminary results of 1 such study have been reported.125
What is certain is that tumor resistance will develop. This last conclusion is supported by the observation that nearly all patients with HER2-positive metastatic breast cancer appear to become refractory within 12 months after initially responding to trastuzumab.104 The finding that most of the patients (with tumors resistant to trastuzumab) exhibit disease progression after 6 months of treatment with lapatinib suggests that resistance also develops against small-molecule inhibitors. Even when used in the adjuvant setting, disease relapse has occurred following antibody therapy.69,84 As such, research must be directed toward understanding the molecular and biochemical mechanisms of tumor resistance. Elucidating these mechanisms would be beneficial in 2 ways: 1) the development of effective alternative agents and 2) the introduction of strategies that could prevent (or at least delay) the emergence of drug-resistant tumor cells. The former is already underway. One of the most promising new agents is pertuzumab (Omnitarg™), a drug that binds to a critical region on the ectodomain of HER2 thereby interfering with the receptor’s ability to dimerize. Phase I trials have shown pertuzumab to be tolerable and active.126 Preliminary results of a phase II study indicate significant activity in patients who progressed on trastuzumab.127 Of the 33 patients enrolled, 1 complete response, 5 partial responses, and 17 stable disease were achieved. Preclinical studies suggest that pertuzumab can even inhibit growth of tumor cells with low-level expression of HER2.128
What have also been elucidated are the many pathways (and hence targets) downstream, or completely independent, of HER2 that may be linked to the aberrant behavior of HER2-positive tumor cells. Mutations involving PI3K, Akt, and mTOR (mammalian target of rapamycin) may result in constitutive activation of these effectors as well as tumor resistance. As such, new inhibitors such as LY294002 (a quercetin derivative), perifosine (Keryx Pharmaceuticals), and everolimus (Novartis Pharmaceuticals), which have been tested in preclinical models, may have a role in the treatment of patients with HER2-positive disease. In addition, insulin-like growth factor-1 (IGF-1) also appears to be an important co-receptor with the unique ability to induce HER2 phosphorylation.129 Thus, targeted inhibition of insulin-like growth factor-1 may be beneficial in patients with HER2 overexpressing breast cancer. Other types of therapies that are currently being investigated include inhibitors of heat-shock protein 90 and histone deacetylase, irreversible inhibitors of the HER2 kinase, combinations of HER2 inhibitors and antiangiogenic agents, and chemotherapy conjugated to an antibody.
Despite the plethora of publications related to HER2, the extent of what is not known remains uncertain. The reality is that HER2 is an extraordinarily dynamic structure, whose genome will likely be altered by mutational and epigenetic events. Nonetheless, it is highly conceivable that scientists will one day unravel the complex mysteries of tumor cell signaling, may be even HER, too.
Footnotes
Disclosures
The authors disclose no conflicts of interest.
References
- 1.Fisher B, Anderson S, Redmond CK, et al. Re-analysis and results after 12 years of follow-up in a randomized clinical trial comparing total mastectomy with lumpectomy with or without irradiation in the treatment of breast cancer. N Engl J Med. 1995;333(22):1456–1461. doi: 10.1056/NEJM199511303332203. [DOI] [PubMed] [Google Scholar]
- 2.Jacobson JA, Danforth DN, Cowan KH, et al. Ten-year results of a comparison of conservation with mastectomy in the treatment of stage I and II breast cancer. N Engl J Med. 1995;332(14):907–911. doi: 10.1056/NEJM199504063321402. [DOI] [PubMed] [Google Scholar]
- 3.Veronesi U, Salvadori B, Luini A, et al. Breast conservation is a safe method in patients with small cancer of the breast. Long-term results of three randomized trials on 1,973 patients. Eur J Cancer. 1995;31A(10):1574–1579. doi: 10.1016/0959-8049(95)00271-j. [DOI] [PubMed] [Google Scholar]
- 4.Fisher B, Redmond C, Fisher ER, et al. Ten-year results of a randomized clinical trial comparing radical mastectomy and total mastectomy with or without radiation. N Engl J Med. 1985;312(11):674–681. doi: 10.1056/NEJM198503143121102. [DOI] [PubMed] [Google Scholar]
- 5.Krag D, Weaver D, Ashikaga T, et al. The sentinel node in breast cancer: a multicenter validation study. N Engl J Med. 1998;339(14):941–946. doi: 10.1056/NEJM199810013391401. [DOI] [PubMed] [Google Scholar]
- 6.Veronesi U, Paganelli G, Vitale G, et al. Sentinel lymph node biopsy and axillary dissection for breast cancer: results in a large series. J Natl Cancer Inst. 1999;91(4):368–373. doi: 10.1093/jnci/91.4.368. [DOI] [PubMed] [Google Scholar]
- 7.Early Breast Cancer Trialists’ Collaborative Group Tamoxifen for early breast cancer: an overview of the randomised trials. Lancet. 1998;351(9114):1451–1467. [PubMed] [Google Scholar]
- 8.Lippman ME, Dickson RB. Growth control of normal and malignant breast epithelium. Prog Clin Biol Res. 1990;354A:147–178. [PubMed] [Google Scholar]
- 9.Shou J, Massarweh S, Osborne CK, et al. Mechanisms of tamoxifen resistance: increased estrogen receptor-HER2/neu crosstalk in ER/HER2-posivitive breast cancer. J Natl Cancer Inst. 2004;96(12):926–935. doi: 10.1093/jnci/djh166. [DOI] [PubMed] [Google Scholar]
- 10.Xia W, Bacus S, Hegde P, et al. A model of acquired autoresistance to a potent ErbB2 tyrosine kinase inhibitor and a therapeutic strategy to prevent its onset in breast cancer. Proc Natl Acad Sci U S A. 2006;103(20):7795–7800. doi: 10.1073/pnas.0602468103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lonning PE, Taylor PD, Anker G, et al. High-dose estrogen treatment in postmenopausal breast cancer patients heavily exposed to endocrine therapy. Breast Cancer Res Treat. 2001;67(2):111–116. doi: 10.1023/a:1010619225209. [DOI] [PubMed] [Google Scholar]
- 12.von Minckwitz G, Vogel P, Schmidt M, et al. Trastuzumab treatment beyond progression in patients with HER2-positive metastatic breast cancer: the TBP study (GBG 26/BIG 3-05) [abstract] Breast Cancer Res Treat. 2007;106:S185. [Google Scholar]
- 13.Antoine E, Extra J, Vincent-Salomon L, et al. Multiple lines of trastuzumab provide a survival benefit for women with metastatic breast cancer: results from the Hermine cohort study [abstract] Eur J Cancer. 2007;213(Suppl 5):2099. [Google Scholar]
- 14.Downward J, Yarden Y, Mayes E, et al. Close similarity of epidermal growth factor receptor and v-erb-B oncogene protein sequences. Nature. 1984;307(5951):521–527. doi: 10.1038/307521a0. [DOI] [PubMed] [Google Scholar]
- 15.Schechter AL, Stern DF, Vaidyanathan L, et al. The neu oncogene: an erb-B-related gene encoding a 185,000-Mr tumor antigen. Nature. 1984;312(5994):513–516. doi: 10.1038/312513a0. [DOI] [PubMed] [Google Scholar]
- 16.Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2000;103(2):211–225. doi: 10.1016/s0092-8674(00)00114-8. [DOI] [PubMed] [Google Scholar]
- 17.Riese DJ, II, Gallo RM, Settleman J. Mutational activation of ErbB family receptor tyrosine kinases: insights into mechanisms of signal transduction and tumorigenesis. BioEssays. 2007;29(6):558–565. doi: 10.1002/bies.20582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leahy DJ. Structure and function of the epidermal growth factor (EGF/ERB B) family of receptors. Adv Protein Chem. 2004;68:1–27. doi: 10.1016/S0065-3233(04)68001-6. [DOI] [PubMed] [Google Scholar]
- 19.Schlessinger J. Epidermal growth factor receptor pathway. Sci Signal. (Connections Map in the Database of Cell Signaling, as seen 6 September 2009). URL: http://stke.sciencemag.org/cgi/cm/stkecm; CMP_14987.
- 20.Miettinen PJ, Berger JE, Meneses J, et al. Epithelial immaturity and multiorgan failure in mice lacking epidermal growth factor receptor. Nature. 1995;376(6538):337–341. doi: 10.1038/376337a0. [DOI] [PubMed] [Google Scholar]
- 21.Lee KF, Simon H, Chen H, Bates B, Hung MC, Hauser C. Requirement for neuregulin receptor erbB2 in neural and cardiac development. Nature. 1995;378(6555):394–398. doi: 10.1038/378394a0. [DOI] [PubMed] [Google Scholar]
- 22.Jones FE, Stern DF. Expression of dominant-negative ErbB2 in the mammary gland of transgenic mice reveals a role in lobuloaveolar development and lactation. Oncogene. 1999;18(23):3481–3490. doi: 10.1038/sj.onc.1202698. [DOI] [PubMed] [Google Scholar]
- 23.Di Fiore P, Pierce JH, Fleming TP, et al. Overexpression of the human EGF receptor confers an EGF-dependent transformed phenotype to NIH 3T3 cells. Cell. 1987;51(6):1063–1070. doi: 10.1016/0092-8674(87)90592-7. [DOI] [PubMed] [Google Scholar]
- 24.Khazaie K, Dull TJ, Graf T, et al. Truncation of the human EGF receptor leads to differential transforming potentials in primary avian fibroblasts and erythroblasts. EMBO J. 1988;7(10):3061–3071. doi: 10.1002/j.1460-2075.1988.tb03171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harris AL, Nicholson S, Sainsbury R, Wright C, Farndon J. Epidermal growth factor receptor and other oncogenes as prognostic markers. Natl Cancer Inst Monogr. 1992;11:181–187. [PubMed] [Google Scholar]
- 26.Di Marco E, Pierce JH, Fleming TP, et al. Autocrine interaction between TGF alpha and the EGF-receptor: quantitative requirements for induction of the malignant phenotype. Oncogene. 1989;4(7):831–838. [PubMed] [Google Scholar]
- 27.Menard S, Fortis S, Castiglioni F, Agresti R, Balsari A. HER2 as a prognostic factor in breast cancer. Oncology. 2001;61(Suppl 2):67–72. doi: 10.1159/000055404. [DOI] [PubMed] [Google Scholar]
- 28.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ulrich A, McGuire L. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 29.Press MF, Bernstein L, Thomas PA, et al. HER-2/neu gene amplification characterized by fluorescence in situ hybridization: poor prognosis in node-negative breast carcinomas. J Clin Oncol. 1997;15(8):2894–2904. doi: 10.1200/JCO.1997.15.8.2894. [DOI] [PubMed] [Google Scholar]
- 30.Wolff AC, Hammond EH, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25(1):118–145. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 31.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 32.DiGiovanna MP, Stern DF, Edgerton SM, Whalen SG, Moore D, II, Thor AD. Relationship of epidermal growth factor receptor expression to erbB-2 signaling activity and prognosis in breast cancer patients. J Clin Oncol. 2005;23(6):1152–1160. doi: 10.1200/JCO.2005.09.055. [DOI] [PubMed] [Google Scholar]
- 33.Tsutsui S, Ohno S, Murakami S, Kataoka A, Kinoshita J, Hachitanda Y. Prognostic value of the combination epidermal growth factor receptor and c-erbB-2 in breast cancer. Surgery. 2003;133(2):219–221. doi: 10.1067/msy.2003.32. [DOI] [PubMed] [Google Scholar]
- 34.Holbro T, Beerli RR, Maurer F, Koziczak M, Barbas CF, Hynes NE. The ErbB2/ErbB3 heterodimer functions as an oncogenic unit: ErbB2 requires ErbB3 to drive breast tumor cell proliferation. Proc Natl Acad Sci U S A. 2003;100(15):8933–8938. doi: 10.1073/pnas.1537685100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wiseman SM, Makretsov N, Nielsen TO, et al. Coexpression of the type 1 growth factor receptor family members HER-1, HER-2, and HER-3 has a synergistic negative prognostic effect on breast carcinoma survival. Cancer. 2005;103(9):1770–1777. doi: 10.1002/cncr.20970. [DOI] [PubMed] [Google Scholar]
- 36.Witton CJ, Reeves JR, Going JJ, Cooke TG, Bartlett JM. Expression of the HER1-4 family of receptor tyrosine kinases in breast cancer. J Pathol. 2003;200(3):290–297. doi: 10.1002/path.1370. [DOI] [PubMed] [Google Scholar]
- 37.Suo Z, Risberg B, Kalsson MG, et al. EGFR family expression in breast carcinomas, c-erbB-2 and c-erbB4 receptors have different effects on survival. J Pathol. 2002;196(1):17–25. doi: 10.1002/path.1003. [DOI] [PubMed] [Google Scholar]
- 38.Suo Z, Emilsen E, Tveit KM, Nesland JM. Type 1 protein tyrosine kinases in benign and malignant breast lesions. Histopathology. 1998;33(6):514–521. doi: 10.1046/j.1365-2559.1998.00498.x. [DOI] [PubMed] [Google Scholar]
- 39.Fuchs IB, Siemer I, Buhler H, et al. Epidermal growth factor receptor changes during breast cancer metastasis. Anticancer Res. 2006;26(6B):4397–4401. [PubMed] [Google Scholar]
- 40.Baselga J, Albanell J, Molina MA, Arribas J. Mechanism of action of trastuzumab and scientific update. Semin Oncol. 2001;28(5 Suppl 16):4–11. doi: 10.1016/s0093-7754(01)90276-3. [DOI] [PubMed] [Google Scholar]
- 41.Yeon CH, Pegram MD. Anti-erbB-2 antibody trastuzumab in the treatment of HER2-amplified breast cancer. lnvest New Drugs. 2005;23(5):391–409. doi: 10.1007/s10637-005-2899-8. [DOI] [PubMed] [Google Scholar]
- 42.Austin CD, De Maziere AM, Pisacane PI, et al. Endocytosis and sorting of ErbB2 and the site of action of acancer therapeutics trastuzumab and geldanamycin. Mol Biol Cell. 2004;15(12):5268–5282. doi: 10.1091/mbc.E04-07-0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lane HA, Beuvink I, Motoyama AB, Daly JM, Neve RM, Hynes NE. ErbB2 potentiates breast tumor proliferation through modulation of p27(Kip1)-Cdk2 complex formation: receptor overexpression does not determine growth dependency. Mol Cell Biol. 2000;20(9):3210–3223. doi: 10.1128/mcb.20.9.3210-3223.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lane HA, Motoyama AB, Beuvink I, Hynes NE. Modulation of p27/Cdk2 complex formation through 4D5-mediated inhibition of HER2 receptor signaling. Ann Oncol. 2001;12(Suppl 1):S21–S22. doi: 10.1093/annonc/12.suppl_1.s21. [DOI] [PubMed] [Google Scholar]
- 45.Petit AM, Rak J, Hung MC, et al. Neutralizing antibodies against epidermal growth factor and ErbB-2/neu receptor tyrosine kinases down-regulate vascular endothelial growth factor production by tumor cells in vitro and in vivo: angiogenic implications for signal transduction therapy of solid tumors. Am J Pathol. 1997;151(6):1523–1530. [PMC free article] [PubMed] [Google Scholar]
- 46.Izumi Y, Xu L, di Tomaso E, Fukumura D, Jain RK. Tumour biology: herceptin acts as an anti-angiogenic cocktail. Nature. 2002;416(6878):279–280. doi: 10.1038/416279b. [DOI] [PubMed] [Google Scholar]
- 47.Pietras RJ, Pegram MD, Finn RS, Maneval DA, Slamon DJ. Remission of human breast cancer xenografts on therapy with humanized monoclonal antibody to HER-2 receptor and DNA-reactive drugs. Oncogene. 1998;17(17):2235–2249. doi: 10.1038/sj.onc.1202132. [DOI] [PubMed] [Google Scholar]
- 48.Molina MA, Codony-Servat J, Albanell J, Rojo F, Arribas J, Baselga J. Trastuzumab (Herceptin), a humanized anti-HER2 receptor monoclonal antibody, inhibits basal and actiated HER2 ectodomain cleavage in breast cancer cells. Cancer Res. 2001;61(12):4744–4749. [PubMed] [Google Scholar]
- 49.Gennari R, Menard S, Fagnoni F, et al. Pilot study of the mechanism of action of preoperative trastuzumab in patients with primary operable breast tumors overexpressing HER2. Clin Cancer Res. 2004;10(17):5650–5655. doi: 10.1158/1078-0432.CCR-04-0225. [DOI] [PubMed] [Google Scholar]
- 50.Menard S, Pupa SM, Campiglio M, et al. Apoptosis induction by trastuzumab: possible role of the core biopsy intervention. J Clin Oncol. 2005;23(28):7238–7240. doi: 10.1200/JCO.2005.02.4679. [DOI] [PubMed] [Google Scholar]
- 51.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytoxicity against tumor targets. Nat Med. 2000;6(4):443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 52.Fan Z, Masui H, Altas I, Mendelsohn J. Blockade of epidermal growth factor receptor function by bivalent and monovalent fragments of C225 anti-epidermal growth factor receptor monoclonal antibodies. Cancer Res. 1993;53(18):4322–4328. [PubMed] [Google Scholar]
- 53.Leyland-Jones B, Gelmon K, Ayoub JP, et al. Pharmacokinetics, safety, and efficacy of trastuzumab administered every three weeks in combination with paclitaxel. J Clin Oncol. 2003;21(21):3965–3971. doi: 10.1200/JCO.2003.12.109. [DOI] [PubMed] [Google Scholar]
- 54.Wood ER, Truesdale AT, McDonald OB, et al. A unique structure for epidermal growth factor receptor bound to GW572016 (Lapatinib): relationships among protein conformation, inhibitor off-rate, and receptor activity in tumor cells. Cancer Res. 2004;64(18):6652–6659. doi: 10.1158/0008-5472.CAN-04-1168. [DOI] [PubMed] [Google Scholar]
- 55.Takai Y, Sasaki T, Matozaki T. Small GTP-binding proteins. Physiol Rev. 2001;81(1):153–208. doi: 10.1152/physrev.2001.81.1.153. [DOI] [PubMed] [Google Scholar]
- 56.Martinez-Lorenzo MJ, Anel A, Monleon I, et al. Tyrosine phosphorylation of the p85 subunit of phosphatidylinositol 3-kinase correlates with high proliferation rates in sublines derived from the Jurkat leukemia. Int J Biochem Cell Biol. 2000;32(4):435–445. doi: 10.1016/s1357-2725(99)00142-9. [DOI] [PubMed] [Google Scholar]
- 57.Datta SR, Dudek H, Tao X, et al. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91(2):231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 58.Avruch J, Khokhlatchev A, Kyriakis JM, et al. Ras activation of the Raf kinase: tyrosine kinase recruitment of the MAPK cascade. Recent Prog Horm Res. 2001;56:127–155. doi: 10.1210/rp.56.1.127. [DOI] [PubMed] [Google Scholar]
- 59.Ewer MS, Vooletich MT, Durand JB, et al. Reversibility of trastuzumab-related cardiotoxicity: new insights based on clinical course and response to medical treatment. J Clin Oncol. 2005;23(31):7820–7826. doi: 10.1200/JCO.2005.13.300. [DOI] [PubMed] [Google Scholar]
- 60.Gonzalez-Angulo AM, Hortobagyi GN, Esteva FJ. Adjuvant therapy with trastuzumab for HER-2/neu-positive breast cancer. Oncologist. 2006;11(8):857–867. doi: 10.1634/theoncologist.11-8-857. [DOI] [PubMed] [Google Scholar]
- 61.Zhao Y, Sawyer DR, Baliga RR, et al. Neuregulins promote survival and growth of cardiac myocytes. J Biol Chem. 1998;273(17):10261–10269. doi: 10.1074/jbc.273.17.10261. [DOI] [PubMed] [Google Scholar]
- 62.Sawyer DB, Zuppinger C, Miller TA, Eppenberger HM, Suter TM. Modulation of anthracycline-induced myofibrillar disarray in rat ventricular myocytes by neuregulin-1β and anti-erbB2: potential mechanism for trastuzumab-induced cardiotoxicity. Circulation. 2002;105(13):1551–1554. doi: 10.1161/01.cir.0000013839.41224.1c. [DOI] [PubMed] [Google Scholar]
- 63.Negro A, Brar BK, Lee K-F. Essential roles of Her2/erbB2 in cardiac development and function. Recent Progress Hormone Res. 2004;59:1–12. doi: 10.1210/rp.59.1.1. [DOI] [PubMed] [Google Scholar]
- 64.Guarneri V, Lenihan DJ, Valero V, et al. Long term cardiac tolerability of trastuzumab in metastatic breast cancer: The MD Anderson Center experience. J Clin Oncol. 2006;24(25):4107–4115. doi: 10.1200/JCO.2005.04.9551. [DOI] [PubMed] [Google Scholar]
- 65.Kelly H, Kimmick G, Dees EC, et al. Response and cardiac toxicity of trastuzumab given in conjunction with weekly paclitaxel after doxorubicin/cyclophosphamide. Clin Breast Cancer. 2006;7(3):237–243. doi: 10.3816/CBC.2006.n.035. [DOI] [PubMed] [Google Scholar]
- 66.Buzdar AU, Valero V, Ibrahim NK, et al. Neoadjuvant therapy with paclitaxel followed by 5-fluorouracil, epirubicin, and cyclophosphamide chemotherapy and concurrent trastuzumab in human epidermal growth factor receptor 2-positive operable breast cancer: an update of the initial randomized study population and data of additional patients treated with the same regimen. Clin Cancer Res. 2007;13(1):228–233. doi: 10.1158/1078-0432.CCR-06-1345. [DOI] [PubMed] [Google Scholar]
- 67.Dang C, Fornier M, Sugarman S, et al. The safety of dose-dense doxorubicin and cyclophosphamide followed by paclitaxel with trastuzumab in HER-2/neu overexpressed/amplified breast cancer. J Clin Oncol. 2008;26(8):1216–1222. doi: 10.1200/JCO.2007.12.0733. [DOI] [PubMed] [Google Scholar]
- 68.Marty M, Baselga J, Gatzemeier U, et al. Pooled analysis of six trials of trastuzumab (Herceptin): exploratory analysis of changes in left ventricular ejection fraction (LVEF) as a surrogate for clinical events [abstract] Breast Cancer Res Treat. 2003;82:218. [Google Scholar]
- 69.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353(16):1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 70.Perez EA, Byrne JA, Hammond IW, et al. Cardiac safety experience in 3127 patients treated with lapatinib [abstract] Ann Oncol. 2006;17(Suppl 9):1420. [Google Scholar]
- 71.Perez EA, Suman VJ, Davidson N, et al. Cardiac safety analysis of doxorubicin and cyclophosphamide followed by paclitaxel with or without trastuzumab in the North Central Cancer treatment group N9831 adjuvant breast cancer trial. J Clin Oncol. 2008;26(8):1231–1238. doi: 10.1200/JCO.2007.13.5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355(26):2733–2743. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 73.Tan-Chiu E, Yothers G, Romond E, et al. Assessment of cardiac dysfunction in a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel, with or without trastuzumab as adjuvant therapy in node-positive, human epidermal growth factor receptor 2-overexpressing breast cancer: NSABP B-31. J Clin Oncol. 2005;23(31):7811–7819. doi: 10.1200/JCO.2005.02.4091. [DOI] [PubMed] [Google Scholar]
- 74.Popat S, Smith IE. Therapy insight: anthracyclines and trastuzumab – the optimal management of cardiotoxic side effects. Nat Clin Pract Oncol. 2008;5(6):324–335. doi: 10.1038/ncponc1090. [DOI] [PubMed] [Google Scholar]
- 75.Spector NL, Yarden Y, Smith B, et al. Activation of AMP-activated protein kinase by human EGF receptor 2/EGF receptor tyrosine kinase inhibitor protects cardiac cells. Proc Natl Acad Sci U S A. 2007;104(25):10607–10612. doi: 10.1073/pnas.0701286104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vogel CL, Cobleigh MA, Tripathy D, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20(3):719–726. doi: 10.1200/JCO.2002.20.3.719. [DOI] [PubMed] [Google Scholar]
- 77.Baselga J, Carbonell X, Castaneda-Soto NJ, et al. Phase II study of efficacy, safety, and pharmacokinetics of trastuzumab monotherapy administered on a 3-weekly schedule. J Clin Oncol. 2005;23(10):2162–2171. doi: 10.1200/JCO.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 78.Pegram MD, Konecny GE, O’Callaghan C, Beryt M, Pietras R, Slamon DJ. Rational combinations of trastuzumab with chemotherapeutic drugs used in the treatment of breast cancer. J Natl Cancer Inst. 2004;96(10):739–749. doi: 10.1093/jnci/djh131. [DOI] [PubMed] [Google Scholar]
- 79.Gasparini G, Gion M, Mariani L, et al. Randomized phase II trial of weekly paclitaxel alone versus trastuzumab plus weekly paclitaxel as first-line therapy of patients with HER-2 positive advance breast cancer. Breast Cancer Res Treat. 2007;101(3):355–365. doi: 10.1007/s10549-006-9306-9. [DOI] [PubMed] [Google Scholar]
- 80.Pegram MD, Lopez A, Konecny G, et al. Trastuzumab and chemotherapeutics: drug interactions and synergies. Semin Oncol. 2000;27(6 Suppl 11):21–25. [PubMed] [Google Scholar]
- 81.Esteva F, Valero V, Booser D, et al. Phase II study of weekly docetaxel and trastuzumab for patients with HER-2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20(7):1800–1808. doi: 10.1200/JCO.2002.07.058. [DOI] [PubMed] [Google Scholar]
- 82.Marty M, Cognetti F, Maraninchi D, et al. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive meta-static breast cancer administered as first-line treatment: the M77001 study group. J Clin Oncol. 2005;23(19):4265–4274. doi: 10.1200/JCO.2005.04.173. [DOI] [PubMed] [Google Scholar]
- 83.Di Leo A, Gomez HL, Aziz Z, et al. Phase III, double-blind, randomized study comparing lapatinib plus paclitaxel with placebo plus paclitaxel as first-line treatment for metastatic breast cancer. J Clin Oncol. 2008;26(34):5544–5552. doi: 10.1200/JCO.2008.16.2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353(16):1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 85.Smith I, Procter M, Gelber RD, et al. 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: a randomized controlled trial. Lancet. 2007;369(9555):29–36. doi: 10.1016/S0140-6736(07)60028-2. [DOI] [PubMed] [Google Scholar]
- 86.Joensuu H, Kellokumpu-Lehtinen PL, Bono P, et al. Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. N Engl J Med. 2006;354(8):809–820. doi: 10.1056/NEJMoa053028. [DOI] [PubMed] [Google Scholar]
- 87.Buzdar AU, Ibrahim NK, Francis D, et al. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol. 2005;23(16):3676–3685. doi: 10.1200/JCO.2005.07.032. [DOI] [PubMed] [Google Scholar]
- 88.Coudert BP, Arnould L, Moreau L, et al. Pre-operative systemic (neo-adjuvant therapy with trastuzumab and docetaxel for HER2-overexpressing stage II or III breast cancer: results of a multicenter phase II trial. Ann Oncol. 2006;17(3):409–414. doi: 10.1093/annonc/mdj096. [DOI] [PubMed] [Google Scholar]
- 89.Gianni L, Eiermann W, Semiglazov V, et al. Neoadjuvant trastuzumab in patients with HER2-positive locally advanced breast cancer: Primary efficacy analysis of the NOAH trial [abstract] Proc SABC. 2008;31 [Google Scholar]
- 90.Johnston S, Trudeau M, Kaufman B, et al. Phase II study of predictive biomarker profiles for response targeting human epidermal growth factor receptor 2 (HER-2) in advanced inflammatory breast cancer with lapatinib monotherapy. J Clin Oncol. 2008;26(7):1066–1072. doi: 10.1200/JCO.2007.13.9949. [DOI] [PubMed] [Google Scholar]
- 91.Robert N, Leyland-Jones B, Asmar L, et al. Randomized phase III study of trastuzumab, paclitaxel, and carboplatin compared with trastuzumab and paclitaxel in women with HER-2-overexpressing metastatic breast cancer. J Clin Oncol. 2006;24(18):2786–2792. doi: 10.1200/JCO.2005.04.1764. [DOI] [PubMed] [Google Scholar]
- 92.Slamon D, Eiermann W, Pienkowski T, et al. Phase III randomized trial of doxorubicin and cyclophosphamide followed by docetaxel with doxorubicin and clyclophosphamide followed by docetaxel and trastuzumab with docetaxel, carboplatin and trastuzumab in HER 2-positive early breast cancer patients: BCIRG 006 Study [abstract] Proc SABC. 2005;1 [Google Scholar]
- 93.Burstein HJ, Harris LN, Marcom PK, et al. Trastuzumab and vinorelbine as first-line therapy for HER2-overexpressing metastatic breast cancer: multicenter phase II trial with clinical outcomes, analysis of serum tumor markers as predictive factors, and cardiac surveillance algorithm. J Clin Oncol. 2003;21(15):2889–2895. doi: 10.1200/JCO.2003.02.018. [DOI] [PubMed] [Google Scholar]
- 94.O’Shaughnessy JA, Vukelja S, Marsland T, Kimmel G, Ratman S, Pippen JE. Phase II study of trastuzumab plus gemcitabine in chemotherapy-pretreated patients with metastatic breast cancer. Clin Breast Cancer. 2004;5(2):142–147. doi: 10.3816/cbc.2004.n.019. [DOI] [PubMed] [Google Scholar]
- 95.Bartsch R, Wenzel C, Altorjai G, et al. Capecitabine and trastuzumab in heavily pretreated patients with metastatic breast cancer. J Clin Oncol. 2007;25(25):3853–3858. doi: 10.1200/JCO.2007.11.9776. [DOI] [PubMed] [Google Scholar]
- 96.Lin NU, Carey LA, Liu MC, et al. Phase II trial of lapatinib for brain metastases in patients with human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2008;26(12):1993–1999. doi: 10.1200/JCO.2007.12.3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Burgess AW, Cho HS, Eigenbrot C, et al. An open-and-shut case? Recent insights into the activation of EGF/ErbB receptors. Mol Cell. 2003;12(3):541–552. doi: 10.1016/s1097-2765(03)00350-2. [DOI] [PubMed] [Google Scholar]
- 98.Garrett TP, McKern NM, Lou M, et al. The crystal structure of a truncated ErbB2 ectodomain reveals an active conformation, poised to interact with other ErbB receptors. Mol Cell. 2003;11(2):495–505. doi: 10.1016/s1097-2765(03)00048-0. [DOI] [PubMed] [Google Scholar]
- 99.Di Fiore PP, Pierce JH, Kraus MH, Segatto O, King CR, Aaronson SA. erbB-2 is a potent oncogene when overexpressed in NIH/3T3 cells. Science. 1987;237(4811):178–182. doi: 10.1126/science.2885917. [DOI] [PubMed] [Google Scholar]
- 100.Graus-Porta D, Beerli RR, Daly JM, Hynes NE. ErbB-2, the preferred heterodimerization partner of all ErbB receptors, is a mediator of lateral signaling. EMBO J. 1997;16(7):1647–1655. doi: 10.1093/emboj/16.7.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Olayioye MA, Graus-Porta D, Beerli RR, Rohrer J, Gay B, Hynes NE. ErbB-1 and erbB-2 acquire distinct signaling properties dependent upon their dimerization partner. Mol Cell Biol. 1998;18(9):5042–5051. doi: 10.1128/mcb.18.9.5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Alimandi M, Romano A, Curia MC, et al. Cooperative signaling of erbB3 and erbB2 in neoplastic transformation and human mammary carcinomas. Oncogene. 1995;10(9):1813–1821. [PubMed] [Google Scholar]
- 103.Kokai Y, Myers JN, Wada T, et al. Synergistic interaction of p185c-neu and the EGF receptor leads to transformation of rodent fibroblasts. Cell. 1989;58(2):287–292. doi: 10.1016/0092-8674(89)90843-x. [DOI] [PubMed] [Google Scholar]
- 104.Montemurro F, Donadio M, Clavarezza M, et al. Outcome of patients with HER2-positive advanced breast cancer progressing during trastuzumab-based therapy. Oncologist. 2006;11(4):318–324. doi: 10.1634/theoncologist.11-4-318. [DOI] [PubMed] [Google Scholar]
- 105.Bartsch R, Wenzel C, Hussian D, et al. Analysis of trastuzumab and chemotherapy in advanced breast cancer after the failure of at least one earlier combination: an observational study. BMC Cancer. 2006;6:63–68. doi: 10.1186/1471-2407-6-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Garcia-Saenz JA, Martin M, Puente J, et al. Trastuzumab associated with successive cytotoxic therapies beyond disease progression in metastatic breast cancer. Clin Breast Cancer. 2005;6(4):325–329. doi: 10.3816/CBC.2005.n.035. [DOI] [PubMed] [Google Scholar]
- 107.Gelmon KA, Mackey J, Verma S, et al. Use of trastuzumab beyond disease progression: observations from a retrospective review of case histories. Clin Breast Cancer. 2004;5(1):52–58. doi: 10.3816/cbc.2004.n.010. [DOI] [PubMed] [Google Scholar]
- 108.Xia W, Liu LH, Ho P, Spector NL. Truncated ErbB2 receptor (p95ErbB2) is regulated by heregulin through heterodimer formation with ErbB3 yet remains sensitive to the dual EGFR/ErbB2 kinase inhibitor GW72016. Oncogene. 2004;23(3):646–653. doi: 10.1038/sj.onc.1207166. [DOI] [PubMed] [Google Scholar]
- 109.Segatto O, King CR, Pierce JH, DiFiore PP, Aaronson SA. Different structural alterations upregulate in vitro tyrosine kinase activity and transforming potency of the erbB-2 gene. Mol Cell Biol. 1988;8(12):5570–5574. doi: 10.1128/mcb.8.12.5570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Colomer R, Montero S, Lluch A, et al. Circulating HER2 extracellular domain and resistance to chemotherapy in advanced breast cancer. Clin Cancer Res. 2000;6(6):2356–2362. [PubMed] [Google Scholar]
- 111.Molina MA, Saez R, Ramsey, et al. NH(2)-terminal truncated HER-2 protein but not full-length receptor is associated with nodal metastasis in human breast cancer. Clin Cancer Res. 2002;8(2):347–353. [PubMed] [Google Scholar]
- 112.Moulder SL, Yakes FM, Muthuswamy SK, Bianco R, Simpson JF, Arteaga CL. Epidermal growth factor receptor (HER1) tyrosine kinase inhibitor ZD1839 (Iressa) inhibits HER2/neu (erbB2)-overexpressing breast cancer cells in vitro and in vivo. Cancer Res. 2001;61(24):8887–8895. [PubMed] [Google Scholar]
- 113.Hirata A, Hosoi F, Miyagawa M, et al. HER2 overexpression increases sensitivity to gefitinib, an epidermal growth factor receptor tyrosine kinase inhibitor, through inhibition of HER2/HER3 heterodimer formation in lung cancer cells. Cancer Res. 2005;65(10):4253–4260. doi: 10.1158/0008-5472.CAN-04-2748. [DOI] [PubMed] [Google Scholar]
- 114.Anido J, Matar P, Albanell, et al. ZD1839, a specific epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor, induces the formation of inactive EGFR/HER2 and EGFR/HER3 heterodimers and prevents heregulin signaling in HER-overexpressing breast cancer cells. Clin Cancer Res. 2003;9(4):1274–1283. [PubMed] [Google Scholar]
- 115.Meyer D, Birchmeier C. Multiple essential functions of neuregulin in development. Nature. 1995;378(6555):386–390. doi: 10.1038/378386a0. [DOI] [PubMed] [Google Scholar]
- 116.Erickson SL, O’Shea KS, Ghaboosi N, et al. ErbB3 is required for normal cerebellar and cardiac development: a comparison of ErbB2-and heregulin-deficient mice. Development. 1997;124(24):4999–5011. doi: 10.1242/dev.124.24.4999. [DOI] [PubMed] [Google Scholar]
- 117.Nicholson RI, Hutcheson IR, Knowlden JM, et al. Nonendocrine pathways and endocrine resistance; observations with antiestrogens and signal transduction inhibitors in combination. Clin Cancer Res. 2004;10(1 Pt 1):346–354s. doi: 10.1158/1078-0432.ccr-031206. [DOI] [PubMed] [Google Scholar]
- 118.Tang CK, Perez C, Grunt T, Waibel C, Cho C, Lupu R. Involvement of heregulin-beta2 in the acquisition of the hormone-independent phenotype of breast cancer cells. Cancer Res. 1996;56(14):3350–3358. [PubMed] [Google Scholar]
- 119.Knowlden JM, Hutcheson IR, Jones HE, et al. Elevated levels of epidermal growth factor receptor/cerB2 heterodimers mediate an autocrine growth regulatory pathway in tamoxifen-resistant MCF-7 cells. Endocrinology. 2003;144(3):1032–1044. doi: 10.1210/en.2002-220620. [DOI] [PubMed] [Google Scholar]
- 120.Nicholson RI, Staka C, Boyns F, Hutcheson IR, Gee JM. Growth factor-driven mechanisms associated with resistance to estrogen deprivation in breast cancer: new opportunities for therapy. Endocr Relat Cancer. 2004;11(4):623–641. doi: 10.1677/erc.1.00778. [DOI] [PubMed] [Google Scholar]
- 121.Johnston S, Pegram M, Press M, et al. Lapatinib combined with letrozole vs letrozole alone for front-line postmenopausal hormone receptor positive metastatic breast cancer: first results from the EGF30008 trial [abstract 46]. 31st Annual San Antonio Breast Cancer Symposium; December 10–14, 2008. [Google Scholar]
- 122.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: Report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90(18):1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 123.Vogel GV, Costantino JP, Wickerham DL, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: The NSABP study of tamoxifen and raloxifene (STAR) P-2 trial. JAMA. 2006;295(23):2727–2741. doi: 10.1001/jama.295.23.joc60074. [DOI] [PubMed] [Google Scholar]
- 124.Cuzick J, Forbes J, Edwards R, et al. First results from the International Breast Cancer Intervention Study (IBIS-1): a randomised prevention trial. Lancet. 2002;360(9336):817–824. doi: 10.1016/s0140-6736(02)09962-2. [DOI] [PubMed] [Google Scholar]
- 125.Swaby R, Blackwell K, Jiang Z, et al. Neratinib in combination with trastuzumab for the treatment of advanced breast cancer: a phase I/II study [abstract 1004] J Clin Oncol. 2009;27(15S):42s. [Google Scholar]
- 126.Agus DB, Gordon MS, Taylor C, et al. Phase I clinical study of pertuzumab, a novel HER dimerization inhibitor, in patients with advanced cancer. J Clin Oncol. 2005;23(11):2534–2543. doi: 10.1200/JCO.2005.03.184. [DOI] [PubMed] [Google Scholar]
- 127.Fumoleau P, Wardley A, Miles D, et al. Safety of pertuzumab plus trastuzumab in a phase II trial of patients with HER2-overexpressing metastatic breast cancer which had progressed during trastuzumab therapy [abstract 73]. 30th Annual San Antonio Breast Cancer Symposium; December 13–16, 2007. [Google Scholar]
- 128.Agus DB, Akita RW, Fox WD, et al. Targeting ligand-activated ErbB2 signaling inhibits breast and prostate tumor growth. Cancer Cell. 2002;2(2):127–137. doi: 10.1016/s1535-6108(02)00097-1. [DOI] [PubMed] [Google Scholar]
- 129.Nahta R, Yuan LXH, Bing Zhang, Kobayashi R, Esteva FJ. Insulin-like growth factor-1 receptor/human epidermal growth factor receptor 2 heterodimerization contributes to trastuzumab resistance of breast cancer cells. Cancer Res. 2005;65(23):11118–11128. doi: 10.1158/0008-5472.CAN-04-3841. [DOI] [PubMed] [Google Scholar]