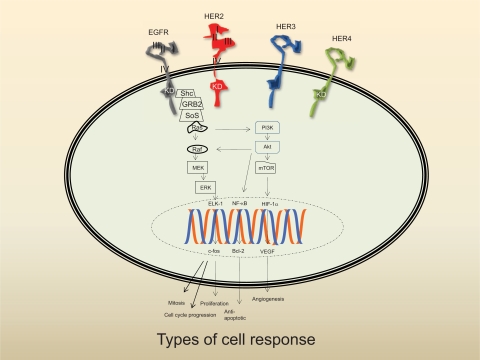
Figure 1.
Schema of the HER family signaling pathways. The linear pathway whereby each component merely functions as a relay switch is grossly oversimplified. The ultimate cellular response is dependent on a diverse array of signals, which is mediated by receptor cross-talk, feedback loops, and counter-regulatory activities. Following ligand binding, the receptor dimerizes and is then phosphorylated. The Ras pathway is especially complex as the kinase must undergo post-translational modification before it can be activated. Phosphorylated HER2 is linked to the Ras signal transduction pathway by binding to 2 adaptor molecules (Shc) and growth-factor receptor bound protein 2 (GRB2). GRB2 forms a complex with son of sevenless (SoS) which is recruited to the plasma membrane leading to activation of Ras. Extracellular signals are propagated from cell surface to nucleus through dynamic interactions with components located downstream of the receptor including PI3K, Akt, and nuclear factor (NF-κβ). Akt blocks apoptosis by antagonizing a proapoptotic member of the Bcl-2 family, Bad, or upregulating Bcl-XL, an anti-apoptotic member of the Bcl-2 family. Akt can also effect angiogenesis via mTor-induced hypoxia inducible factor-1 alpha (HIF-1α) activation. Other downstream effectors of the Ras signal transduction pathway include soluble Raf, mitogen-activated protein kinase kinase (MEK), ERK, and the transcription factors Elk-1 and c-Fos, a nuclear proto-oncogene that regulates expression of cyclin-dependent kinases (CdK).
Notes: 1) domains I and III are believed to be the primary ligand binding site. The “extended” or closed conformer of HER2 precludes ligand access; 2) the absence of the kinase domain (KD) on HER3 represents the impairment of this receptor; and 3) PI3K preferentially binds to phospho-HER3.