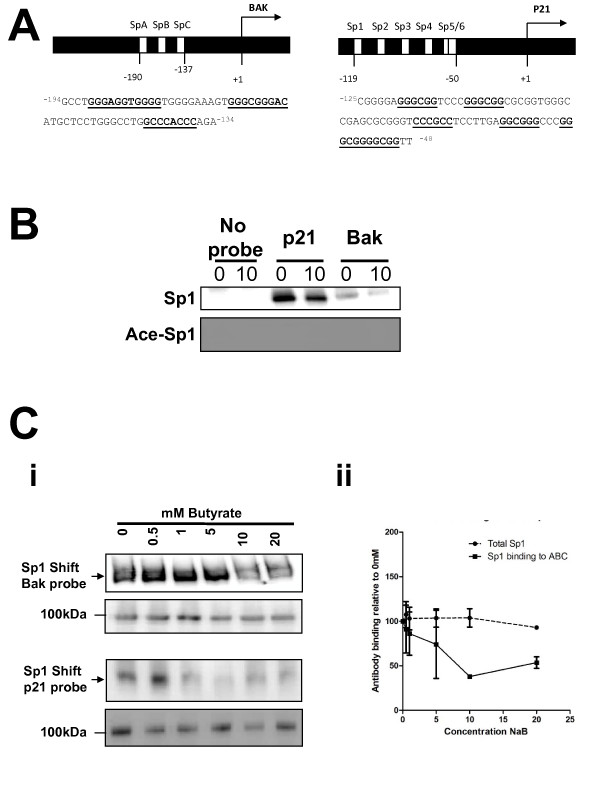
Figure 2.
Acetylated Sp1 does not bind at the bak or p21 promoters. Panel A shows the organisation and sequence of the bak (Ai) and p21 (Aii) probes, with hypothetical and proven Sp1/3 binding sites underlined. Sequence numbers refer to distance from the transcriptional start site of each gene. Panel B shows that binding of Sp1 to both target sequences is decreased following butyrate treatment. Panel B: Western of mobility shift assay (WeMSA) analysis of binding to the Bak and p21 probes shows that Sp1 binding decreases following treatment with 10 mM sodium butyrate for 24 h compared to an untreated control (upper panel). Binding of acetyl-Sp1 could not be detected by WeMSA (lower panel). These data are representative of three independent repeats. Panel C: The binding of Sp1 to the bak (panel Ci) and p21 (panel Cii) promoter sequences was determined following treatment of HCT116 cells with a range of butyrate concentrations (0-20 mM). The upper panels show WeMSA gels immunoprobed for Sp1. As a loading control, the same extracts were also separated by SDS page, and immunoprobed with the same antibody (lower panels). Data shown in Ci and Cii are representative of at least two independent repeat experiments. Panel Ciii shows mean (+/- SD) of response at the Bak promoter. Whilst the levels of Sp1 are broadly constant, levels of Sp1 binding for both probes are reduced following treatment with butyrate.