During orthotopic liver transplantation in normal dogs, it is necessary, at the time that the portal vein and inferior vena cava are cross-clamped, to shunt the venous return from these systems back to the heart in order to prevent cardiovascular collapse.1,4 In man, who is probably naturally endowed with a richer collateral circulation and who has additional venous pathways secondary to liver disease, this has not been necessary,3 and our clinical impression has been that the interruption of venous return has been reasonably well tolerated.
The present study was done to quantitate the changes in hemodynamics in six patients during orthotopic liver transplantation. To our knowledge, this kind of information has not been previously reported.
METHODS
Hemodynamic measurements were performed on six patients (Table I) who underwent hepatic transplantation. Two were children, both aged 3½ years, with biliary atresia. Two were adolescents (aged 15½ and 16 years), of whom one had Wilson’s disease and end-stage cirrhosis and the other had chronic aggressive hepatitis with hepatic failure. The other two patients were adults, both 47 years old, with Laennec’s cirrhosis (Table I).
Table I.
Liver recipients
| Blood |
|||||||
|---|---|---|---|---|---|---|---|
| Patient | Age (yr) | Disease | Date of operation | Ascites (c. c.) | Loss (c. c.) | Replacement (c. c.) | Fluid replacement (c. c.) |
| E. S. | 47 | Laennec’s cirrhosis | 1/16/70 | 5,000 | 6,700 | 7,500 | 1,500 |
| S. N. | 3½ | Biliary atresia | 9/19/70 | Little | 3,750 | 4,000 | 5,700 |
| K. P. | 3½ | Biliary atresia | 9/22/70 | Little | 1,500 | 1,750 | 2,750 |
| P. P. | 47 | Laennec’s cirrhosis | 10/8/70 | 13,000 | 6,500 | 6,500 | 13,700 |
| C. E. | 16 | Chronic aggressive hepatitis (cirrhosis) | 2/27/71 | 10,000 | 8,600 | 10,000 | 15,900 |
| G. W. | 15½ | Wilson’s disease (cirrhosis) | 3/23/71 | Little | 4,000 | 3,800 | 4,800 |
Following induction of anesthesia, catheters were inserted into a radial artery and superior vena cava via a peripheral vein to monitor arterial and central venous pressures and to perform cardiac output studies. Serial hemodynamic measurements were made before, during, and following hepatic transplantation. Cardiac outputs were determined with the use of the dye-dilution technique (cardiogreen) and an output computer.* Other hemodynamics studies included mean arterial blood pressure, central venous pressure, heart rate, stroke volume, and peripheral vascular resistance. These observations were made sequentially (Fig. 1) as follows: (1) before or during laparotomy; (2) during dissection of the recipient’s liver; (3) shortly after cross-clamping the inferior vena cava, portal vein, and hepatic artery (early anhepatic phase); (4) just before revascularization of the new liver (late an hepatic phase); (5) approximately one hour later; and (6) several hours after transplantation with the patient awake. During the operation, fluid and blood losses and changes in electrolytes, acid-base profile, and blood sugar levels were appropriately managed.
Fig. 1.
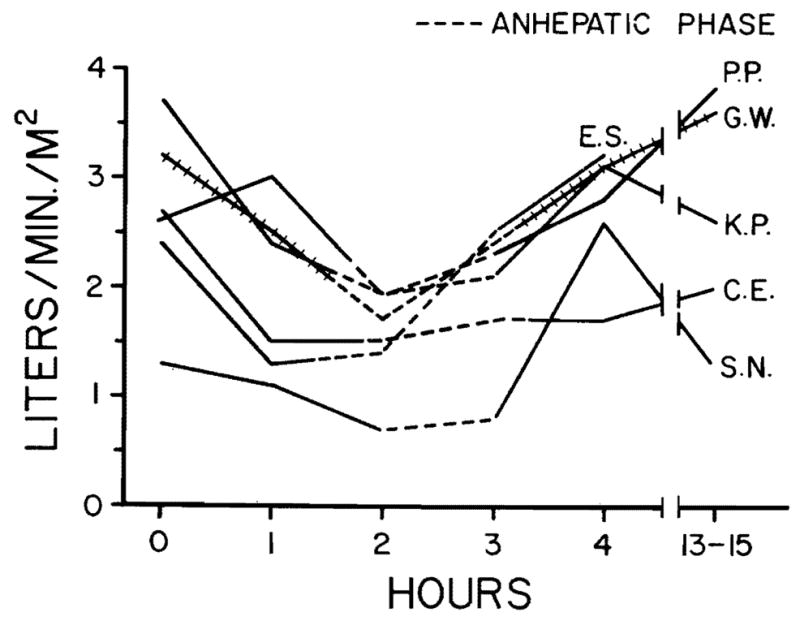
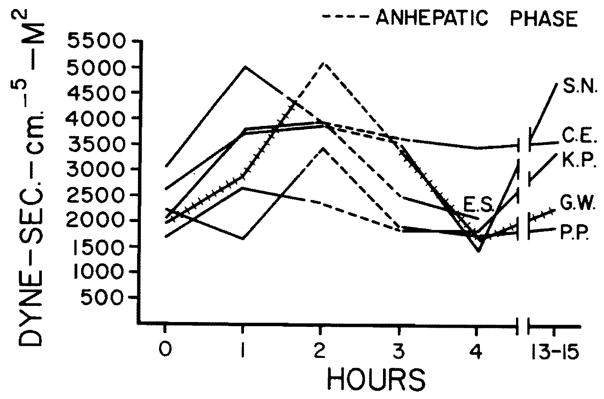
Cardiac indices measured before, during, and following orthotopic liver transplantation in six patients. Note the reduction during the anhepatic phase when the inferior vena cava and portal vein were cross-clamped. The indices returned to prehepatectomy levels following revascularization of the new liver.
RESULTS
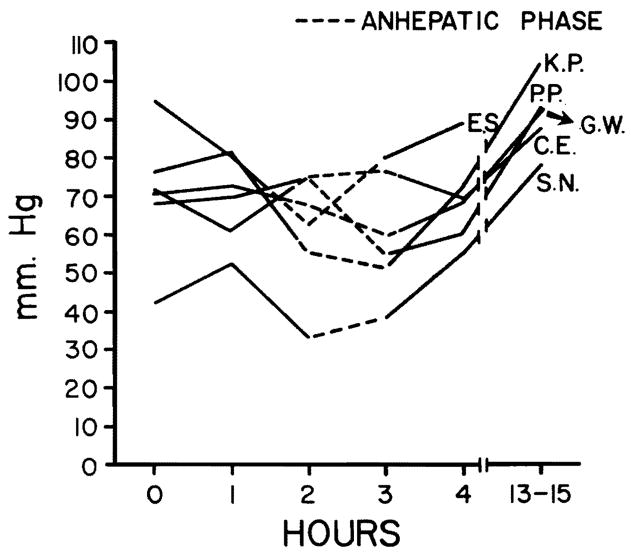
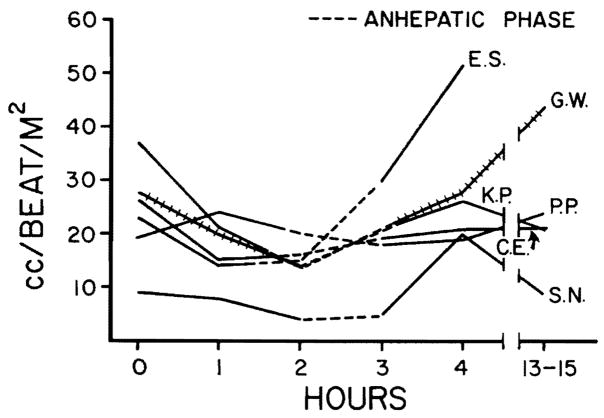
Hemodynamic changes before, during, and following hepatic transplantation are shown in Figs. 1 through 4. Alterations in hemodynamics became apparent before any of the vessels were clamped; these occurred while the host liver was being mobilized. At times, marked displacement of the organ was necessary with resultant interference with venous return. During the anhepatic phase (with clamping of the portal vein and inferior vena cava), the cardiac index, stroke volume index, and mean arterial pressure fell. The maximum mean fall in cardiac index and stroke volume amounted to 43 and 39 percent, respectively, whereas the average fall in mean arterial pressure was 18 percent (Figs. 1 through 3). Simultaneously, the peripheral vascular resistance rose by 71 percent (Fig. 4), but the pulse rate rose only slightly (four percent).
Fig. 4.
The peripheral vascular resistance rose during the anhepatic period but returned to pre-existing levels following the transplantation.
Fig. 3.
In four patients, the mean arterial pressure fell during the anhepatic phase, whereas it rose in two (G. W. and C. E.). There was an average fall in mean arterial pressure of 18 percent during the anhepatic phase.
Following revascularization of the new liver and during the immediate postoperative period, hemodynamic alterations reverted toward pretransplantation levels. Central venous pressure did not change significantly during the operation.
One patient, an extremely ill child (Patient S. N.) had an initial cardiac index of 1.3 L. per minute per square meter, which fell to 0.7 L. per minute per square meter during the anhepatic phase. During revascularization of the new liver, cardiac arrest occurred. This was immediately corrected by external cardiac massage, blood replacement, and administration of inotropic agents.
DISCUSSION
During orthotopic liver transplantation in the dog, interruption of portal venous and inferior caval return to the heart produces cardiovascular collapse. To obviate this complication, it is necessary to shunt blood from both venous systems back to the heart. This can be accomplished with two separate bypass catheters.1 Alternatively, a side-to-side anastomosis may be constructed between the inferior vena cava and superior mesenteric vein, and then a single external Silastic shunt is placed between the external iliac and jugular veins to decompress both venous systems during the anhepatic phase.4
The absolute necessity for effective venous decompression in the dog was erroneously extrapolated to be a mandatory technical condition in the first trials of orthotopic liver transplantation in man, and, in our initial clinical experience, similar shunts to those used in the dog were employed. The bypasses were cumbersome and may have contributed to an unacceptably high incidence of postoperative thromboembolism.3 In all later cases, the portal vein and inferior vena cava were cross-clamped without the aid of any shunts.3 It was found that the procedure was reasonably well tolerated, probably because man has inherently richer collateral veins in the venous system. In addition, patients with chronic liver disease have an even greater anastomotic capability, presumably as a result of an increase in size and number of such collateral veins, a concept that has been shown by Picache and associates2 to pertain in dogs as well.
In the patients during vena caval and portal cross-clamping, a slight duskiness of the intestine has been noted, more so when liver replacement was for hepatoma than when it was for cirrhosis.3 At the same time, a fall in blood pressure was usually noted, which returned to pre-existing levels after the clamps were released. In one particularly interesting case, a child had liver replacement for biliary atresia with essentially no hemodynamic changes. He required a second orthotopic transplant 68 days later. At the second operation, there was very severe hypotension during the anhepatic phase, presumably because of involution of pre-existing collaterals in the intervening two months.3
In all the presently reported patients, portal hypertension and extensive collateral veins were present. Nevertheless, alterations in hemodynamics occurred before the cross-clamping of the venous return from the lower half of the body. At that time, the recipient’s liver was being prepared for excision, and marked displacement of the organ was often required. The altered hemodynamics were probably related to partial interruption of venous return during liver retraction. Moreover, the cardiac output, stroke volume, and mean arterial blood pressure fell even more during occlusion of the portal vein and inferior vena cava; there was a concomitant increase in peripheral vascular resistance. Following revascularization, these potentially serious changes returned to prehepatectomy levels. During the anhepatic phase, fluid and blood replacement were given in excess of loss in anticipation of volume depletion incurred with revascularization of the new liver. This may be responsible for less noticeable changes in hemodynamics during the late anhepatic phase. Cardiac arrest in one patient occurred during revascularization of the new liver, but with successful resuscitation. The incident could probably have been avoided if sufficient blood replacement had been furnished prior to revascularization.
The findings of this study indicate that significant hemodynamic alterations occur in clinical orthotopic transplantation during interruption of venous return from the portal vein and lower half of the body. However, no permanent harmful effects are produced if this period is kept short. It need not be a particularly dangerous part of the operation, provided that there is careful monitoring and appropriate treatment.
SUMMARY
In clinical orthotopic transplantation of the liver, alterations in cardiohemodynamics occurred during interruption of the portal vein and inferior vena cava. The cardiac output, stroke volume, and mean arterial pressure fell by 43, 39, and 18 percent, respectively, with a concomitant rise in peripheral vascular resistance (71 percent) and only a slight increase in heart rate (four percent) during the anhepatic phase. These changes returned to prehepatectomy levels following revascularization of the new liver. Although hemodynamic alterations occur during liver transplantation, with careful monitoring and appropriate therapy, no permanent harmful effects are produced.
Fig. 2.
The changes in stroke volume index in the same six patients paralleled the changes in cardiac index seen in Fig. 1.
Acknowledgments
Aided by research grants from the Veterans Administration, by Grants No. RR-00051 and RR-00069 from the General Clinical Research Centers Program of the Division of Research Resources, National Institutes of Health, and by Grants No. AI-10176-01, AI-AM-08898, AM-07772, and HE-09110 of the United States Public Health Service.
Footnotes
Presented at the Twenty-fifth Annual Meeting of the Society for Vascular Surgery, Philadelphia, Pa., June 18–19, 1971.
Lexington Instruments Corp., Waltham, Mass.
References
- 1.Moore FD, Wheeler HB, Demissianos HV, Smith LL, Balankura O, Abel K, Greenberg JB, Dammin GJ. Experimental whole-organ transplantation of the liver and of the spleen. Ann Surg. 1960;152:374. [PMC free article] [PubMed] [Google Scholar]
- 2.Picache RS, Kapur BML, Starzl TE. The effect of liver disease on the need for venous decompression during the anhepatic phase of canine orthotopic liver transplantation. Surgery. 1970;67:319. [PMC free article] [PubMed] [Google Scholar]
- 3.Starzl TE. Experience in hepatic transplantation. Philadelphia, Pa: W. B. Saunders Company; 1969. (with the assistance of Putnam, C. W.) [Google Scholar]
- 4.Starzl TE, Kaupp HA, Brock DR, Lazarus RE, Johnson RV. Reconstructive problems in canine liver homotransplantation with special reference to the postoperative role of hepatic venous flow. Surg Gynecol Obstet. 1960;111:733. [PMC free article] [PubMed] [Google Scholar]