Abstract
Cisplatin-induced cell death can be triggered by cell-to-cell communication through gap junctions. Here we show that activated src produces tyrosine phosphorylation of the gap junction protein connexin 43, decreases gap junction communication, and increases cell survival in response to cisplatin. Experiments with mixed cell populations show that src activity in one cell can confer increased cisplatin survival on neighboring cells, even when the neighboring cells lack such src activity. This work is the first demonstration that expression of an oncogene in one cell can effect the survival of a neighboring cell not expressing the oncogene in response to a chemotherapeutic drug. The trans-acting effect of activated src on neighboring cells can be blocked by inhibitors of src kinase and counteracted by forced up-regulation of connexin 43, by either gene transfer or proteasome inhibition. These results identify a novel pathway of cisplatin resistance that may be amenable to therapeutic intervention.
Keywords: cisplatin, gap junctions, activated src, connexin 43
Introduction
Once activated in a cell, oncogenes generally promote increased growth and survival. In addition, oncogene expression in cancer cells commonly confers resistance to chemotherapy and radiation. The increased resistance is thought to occur through cell autonomous mechanisms, in which an activated oncogene in a cell confers resistance to that particular cell (1, 2). In this work, we report a novel pathway by which expression of an oncogene, src, in one cell can increase survival of neighboring cells that are not, themselves, expressing the oncogene, to the chemotherapeutic drug, cisplatin.
Prior work by our group identified a pathway of cisplatin toxicity involving cell-to-cell signaling that is dependent on the DNA-dependent protein kinase (DNA-PK) complex and on gap junction intercellular communication (GJIC) (3). In this pathway cisplatin damage in one cell triggers a signal that is dependent on DNA-PK, and is transmitted via GJIC to a neighboring cell to cause cell death. We therefore hypothesized that factors that modulate GJIC might influence the response of cell populations to cisplatin treatment specifically by affecting the cell-to-cell response pathway. Based on a report that connexin 43 (cx43) is a phosphorylation target of src (4), we asked whether activated src can modulate the intercellular transmission of the death signal in response to cisplatin.
To test this, we developed a series of genetically defined cell lines derived from mouse embryonic fibroblasts (MEFs), including wild-type (wt) MEFs, MEFs expressing activated v-Src, and MEFs deficient in Ku80, a key component of the DNA-PK complex. Because prior work established that Ku80-/- MEFs cannot initiate the cisplatin cell-to-cell death signal but can still receive it, they served as receiver cells to examine the trans-acting effects of activated src expression. We report here that expression of activated src in one group of cells attenuates their ability to send a death signal to neighboring cells, thereby conferring resistance to cells in the overall population even if they do not express activated src. In addition, forced overexpression of cx43 by gene transfer or by proteasome inhibition reversed the effect of v-Src, while the use of a src kinase inhibitor blocked it, leading in all three cases to increased sensitivity of neighboring cells to cisplatin. Hence, the work identifies novel pharmacologic strategies to sensitize tumor cells to cisplatin.
Materials and Methods
Cell Lines
wt and Ku80-/- immortalized MEFs were grown in DMEM with 10% FBS. A pcDNA3 vector containing the cDNA for v-Src (generous gift from G. Steven Martin, University of California, Berkeley) was stably transfected into wt MEFs. Positive clones (Src1 and Src2) were selected with 800 μg/ml of geneticin. To create the Src1+cx43 cell line, overexpressing human cx43, wt MEFS were stably transfected with human cx43 cDNA cloned into pcDNA 3.1 and a positive clone was selected using 0.1 mg/ml zeocin.
Drug Treatment and Survival Assays
Cells were treated with indicated concentrations of cisplatin (Sigma) for 1 h. In addition, as indicated, cells were treated with 1 μM PP2 (Calbiochem) for 2 h prior to and concurrent with cisplatin treatment.
High and low density clonogenic survival experiments and monolayer growth assay with mixed populations of cells were performed as previously described (3) with the following alterations. To measure survival exclusively in Ku80-/- cells, we created a puromycin(puro)-resistant cell line by stably transfecting them with the pCMV-puro-bam vector and resistant cells were selected and maintained using 2μg/ml of puro (Sigma). For the experiments with the proteasome inhibitor, MG132 (A.G. Scientific), Src1 cells were pretreated with MG132 (100nM) for 24 h, MG132 was removed, and the cells were trypsinized and seeded 1:1 with Ku80-puro+ cells. The cells were allowed to attach for 3 h, treated with cisplatin, and followed by the monolayer growth assay.
Assays for cell communication
The Lucifer yellow dye transfer assay was performed as previously described (5). The flow cytometry assay was adapted from Czyz et al (6). Briefly, donor cells were trypsinized, resuspended in 0.3M glucose, and pre-loaded for 30 minutes with 50nM calcein AM and 90 nM DiI (Inivitrogen). The pre-loaded donor cells were washed 3 times with PBS, and added to a monolayer of unstained recipient cells of the same type at a ratio of 1:25 (donor:recipient). Donor and recipient cells were co-cultured for 3 h then collected by trypsinization, resuspended in PBS and analyzed immediately on Becton Dickinson FACSCalibur. Data was analyzed by FlowJo software.
Western Blotting and Immunoprecipitation
Cell lysates were collected and processed for western blot as previously described (7). Primary antibodies were: anti-v-Src (Ab-1) (Calbiochem), anti-cx43 (BD Transduction Laboratories), anti-p-cx43 (Tyr-265) (Santa Cruz), and anti-γ-tubulin clone GTU-88 (Sigma). Immunoprecipitation was performed as previously described (8). One mg of total cell lysate was incubated for 2 h with anti-cx43 and immunoprecipitates were subject to gel electrophoresis and probed by western blot with anti-phosphotyrosine (Cell Signaling). Gel images were analyzed using NIH image software.
Results
v-Src expression alters connexin phosphorylation and function
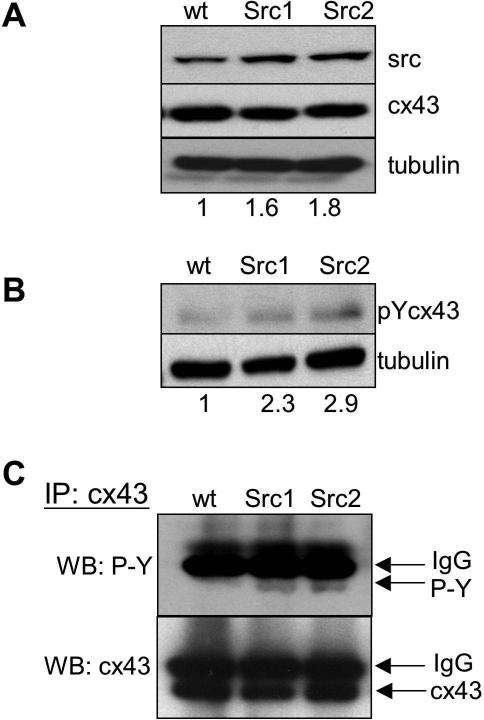
To examine the role of activated src in cisplatin response, we transfected wt MEFs with v-Src cDNA. Stable clones were selected and tested for v-Src expression. Western blotting confirmed that two clones (Src1 and Src2) had increased expression of v-Src above baseline levels of c-Src detected in the parental wt cells (Figure 1A). The antibody recognizes both c-Src and v-Src. Although the overall increase in src levels was found to be only 1.6 and 1.8-fold, the important point is that the additional src expression represented activated v-Src.
Figure 1. v-Src expression mediates connexin phosphorylation.
wt MEFs were transfected with a v-Src expression vector and analyzed for v-Src levels and cx43 phosphorylation. (A) Western blot for src expression in wt MEFs in two subclones, Src1 and Src2. Band intensities for src expression were quantified and normalized to tubulin. The values given below the respective lanes indicate the fold level of expression of src in relation to wt cells. (B) Western blot for tyrosine phosphorylated cx43 using a cx43 phospho-specific antibody (pYcx43). Values below each lane indicate levels of phospho-cx43 relation to wt cells. (C) Immunoblot analysis with anti-phosphotyrosine antibody (upper panel) and anti-cx43 antibody (lower panel) of samples from the indicated cells first immunoprecipitated with anti-cx43 antibody.
To examine the effect of activated src expression on GJIC, we tested for phosphorylation of cx43, which has two potential src phosphorylation targets at tyrosine 247 and 265. Using an antibody specific for tyrosine-phosphorylated cx43, we detected 2 to 3-fold higher phosphorylation of cx43 in the two sub-clones expressing v-Src (Figure 1B). We also immunoprecipitated cx43 from wt, Src1, and Src2 cells using anti-cx43 antibody and performed immunoblot analysis of the samples using phospho-tyrosine (Figure 1C, upper panel) or cx43 antibodies (Figure 1C, lower panel). The novel bands detected by the anti-phospho-tyrosine antibody in the cx43 immunoprecipitation samples from the Src1 and Src2 cells provide further evidence of increased cx43 phosphorylation in the presence of v-Src.
Impact of v-Src on GJIC
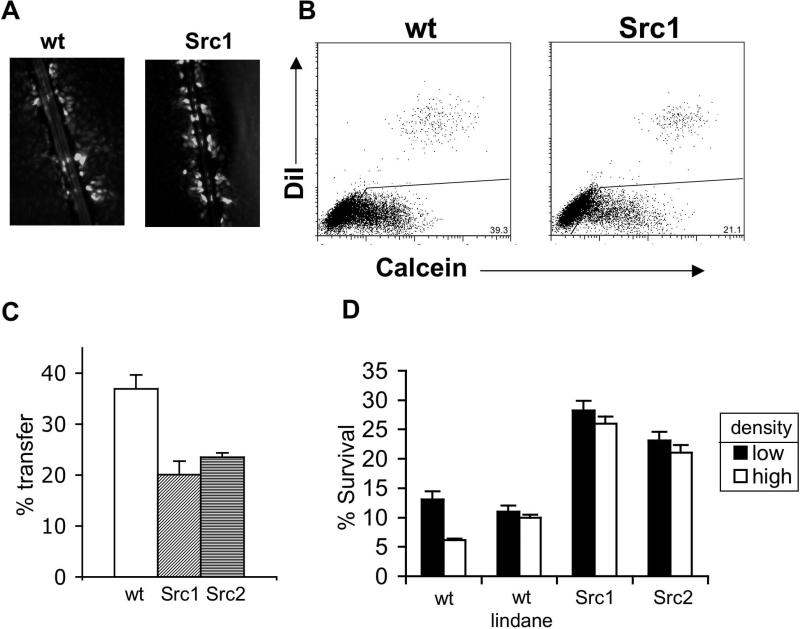
Visualization of GJIC using the technique of Lucifer yellow dye transfer via scrape loading of cell monolayers showed a decrease in GJIC in v-Src expressing clones compared to wt cells (Figure 2A shows data for Src1 compared to wt). To confirm and quantify the change in GJIC caused by v-Src expression, we used a flow cytometry-based assay to assess transfer of calcein dye from cells preloaded with calcein to a population of unloaded cells. As a control, DiI, a fluorescent dye that cannot pass through gap junctions, was also preloaded into the initial cells with calcein. The pre-loaded cells were then washed and mixed with unloaded cells for 3 h, followed by FACS analysis of calcein and DiI content in the mixed population. In the FACS plot (Figure 2B), cells that are positive for both dyes represent preloaded cells, and cells that are positive for only calcein are those that received the calcein dye through GJIC. The expression of v-Src in pre-loaded cells led to a 40% decrease in calcein dye transfer, consistent with decreased GJIC (Figure 2C).
Figure 2. Decreased GJIC and increased survival in v-Src expressing cells.
(A) Visualization of Lucifer Yellow dye transfer after scrape loading in wt and Src1 cells. (B) FACS assay for GJIC. Cells were pre-loaded with 2 fluorescent dyes: calcein, which can pass through gap junctions, and DiI, which cannot. The pre-loaded cells were then overlayed on a monolayer of unstained cells and incubated to allow time for calcein dye transfer via gap junctions. After 3 h, the mixed population was analyzed for calcein and DiI content by FACS. Cells containing both calcein and DiI represent the pre-loaded population. Cells containing only calcein (lower right quadrant) were deemed to have received the dye by GJIC. (C) Quantification of the percentage of unloaded cells that acquired calcein, indicative of gap junction transmission. (D) wt, Src1, or Src2 cells were treated at either high (30,000 cell/cm2) or low (500 cells/cm2) density, as indicated, with 2.5 μg/ml of cisplatin. One set of wt cells was also treated with lindane at 50mg/ml as indicated. For clonogenic survival of cells treated at high density, cells were treated for 1 h at high density, trypsinized and then serially diluted for the colony formation analysis.
v-Src expression abrogates the cell density-dependence of the survival response to cisplatin
As expected, we found that wt MEFs show decreased cisplatin survival at increasing cell densities (Figure 2D), demonstrating the ability of high density cells to engage in cytotoxic cell-to-cell signaling following cisplatin damage (3). The addition of a GJIC inhibitor, lindane, abrogated the density dependence of wt cells, illustrating the role of GJIC in the density effect. With expression of v-Src in the MEFs (Src1 and Src2 cells), the density dependence of the cisplatin response was also eliminated, correlating with the effect of v-Src on GJIC in these cells (Figure 2A-C). Interestingly, the baseline resistance of Src1 and Src2 cells at low density (where there is minimal cell-to-cell communication) was seen to be elevated compared to that of the parental wt MEFs at low density (Figure 2D). This increased survival represents cell autonomous resistance and can be attributed to the effect of activated src on regulatory pathways that act within individual cells to influence cell growth and damage responses.
v-Src increases the cisplatin survival of neighboring cells not expressing the oncoprotein
To further dissect the trans-acting effect of v-Src expression on neighboring cells in high density populations, we took advantage of a previously established cell mixing assay. We had found that even though Ku80-/- cells cannot initiate or send the cell death signal to neighboring cells after treatment with cisplatin, they are able to receive it (3). This finding allowed us to create a system where we can use Ku80-/- cells as a recipient cell population to test the ability of selected MEFs and other defined cell types to send a cytotoxic signal to neighboring cells following cisplatin damage. To specifically measure survival of Ku80-/- cells in mixed populations, a line of Ku80-/- cells was made puro resistant by gene transfer, creating Ku80-puro+ receiver cells.
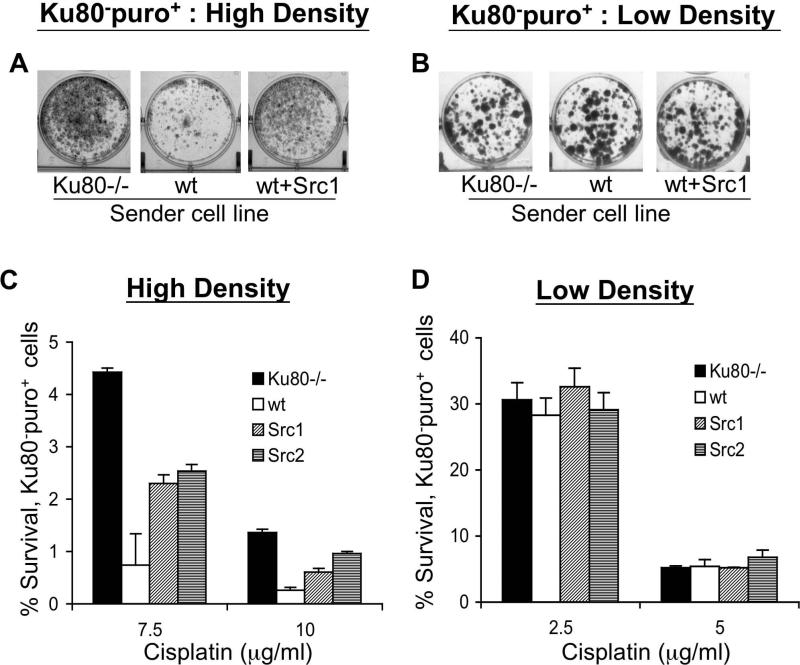
To test the influence of activated src on cell-to-cell signaling in mixed populations, Ku80-/-, wt, Src1 or Src2 cells as “sender cells” were mixed at high or low density with Ku80-puro+ receiver cells, and the mixed populations were treated with cisplatin. Following cisplatin treatment, puro was added to kill all the sender cells and allow for growth of the receiver cell population only. Visualization of monolayer growth of Ku80-puro+ receiver cells showed that, in the high density cultures, the presence of wt sender cells caused greater cisplatin-mediated killing of Ku80-puro+ receiver cells compared to the result in the mixture of Ku80-/- sender cells and Ku80-puro+ receiver cells (Figure 3A). In comparison, the expression of v-Src in wt sender cells (Src1 cells) reduced the cisplatin killing of (and so conferred better survival on) the neighboring Ku80-puro+ cells (Figure 3A). Control experiments established that all sender cells were killed by puro selection and that the visualized monolayer represents only the growth of the Ku80-puro+ cells (data not shown). To confirm that the cells need to be in close contact for v-Src expression in the sender cell population to have an effect on the survival of Ku80-puro+ receiver cells, we repeated the monolayer growth assay with mixed populations of cells seeded at low density (<500 cell/cm2) at the time of cisplatin treatment. At this low density, cells are so spread out on the dish that there is little or no direct contact between neighboring cells and thus gap junction channels are not formed (3). We found that, at low density, there were no differences in survival of the Ku80-puro+ receiver cells regardless of the nature of the co-mixed “sender” cell line (Figure 3B), consistent with a lack of cell-to-cell signaling in these sparse cell populations.
Figure 3. v-Src expression in one cell can influence survival of a neighboring cell not expressing the oncoprotein.
(A and B) The indicated sender cell line was mixed in a 1:1 ratio with puro resistant Ku80-puro+ receiver cells. The mixed populations of cells were treated with cisplatin at (A) high density or (B) low density. After cisplatin exposure, the medium was replaced with fresh medium containing puro to kill off the sender cells and allow only for growth of Ku80-puro+ receiver cells. Seven days after cisplatin treatment, cells were stained with crystal violet and monolayer growth was visualized. (C and D) Colony formation by Ku80-puro+ receiver cells was assayed in mixed population of cells containing Ku80-puro+ cells and indicated sender cells: Ku80-/- MEFs (not puro resistant); wt MEFs; MEF subclones expressing v-Src (Src1 or Src2). The cisplatin treatments were performed with the indicated concentration of drug at high (C) and low (D) overall cell density. Following cisplatin treatment, cell cultures were detached and re-plated in the presence of puro to allow detection and quantification of colony formation specifically by the Ku80-puro+ receiver cells.
To better quantify the above observations, we treated mixed populations at high or low density and then assayed for colony formation of Ku80-puro+ cells following serial dilution. When cells were treated with cisplatin in high density cultures, expression of v-Src in the sender cells (Src1 and Src2) resulted in a more than 3-fold increase in survival of Ku80-puro+ receiver cells compared to when wt cells were mixed with receiver cells (Figure 3C). In contrast, at low density, there was no effect of Src1 or Src2 cells on the survival of Ku80-puro+ cells (Figure 3D). These data show quantitatively that v-Src expression in wt cells can increase survival of neighboring cells to cisplatin even if the neighboring cells do not express the oncoprotein.
The effect of activated src on the survival of neighboring cells is reversed by forced overexpression of cx43 or by src kinase inhibition
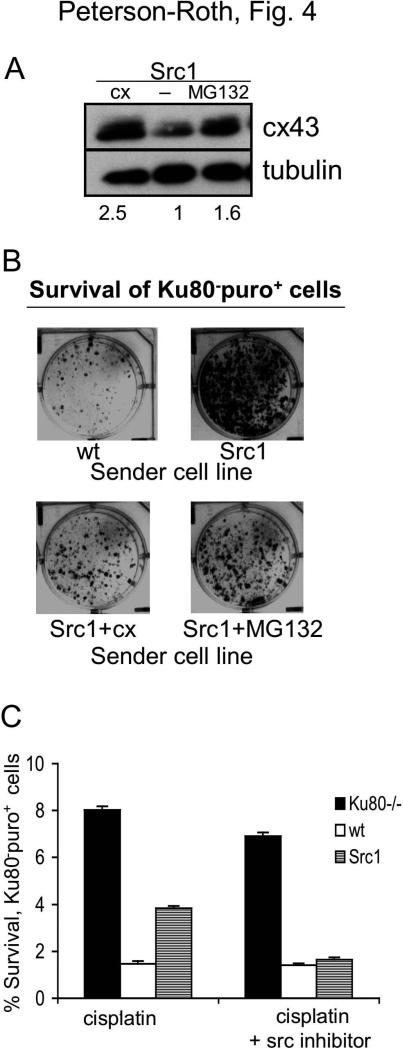
To determine if forced overexpression of cx43 could overcome the effect of v-Src on GJIC and on cell-to-cell cisplatin killing, we used two methods to upregulate cx43 in cells. First, a vector expressing human cx43 cDNA was transfected into Src1 cells, and a clone stably overexpressing cx43 was selected for further study (referred to as Src1+cx) (Figure 4A). Second, it has been reported that blocking cx43 degradation via proteasome inhibition can increase cx43 levels and gap junction formation (9). In our hands, we found that treatment of the Src1 cells for 24 h with the proteasome inhibitor, MG132, led to elevated cx43 expression (Figure 4A), and we determined that the cx43 levels remained high for at least 6 h after MG132 removal (data not shown). Using the monolayer growth assay with Ku80-puro+ receiver cells in mixed populations, we found that there was increased killing of the receiver cells when they were mixed with Src1+cx sender cells or with Src1 sender cells pre-treated with MG132 as compared to unmodified or untreated Src1 cells (Figure 4B). It is important to note that in the Src1+MG132 sample, only Src1 cells were treated with MG132; Ku80-puro+ receiver cells were not exposed to the agent and were mixed with Src1 cells only after MG132 had been removed. This protocol was designed to ensure that survival of the receiver cells was not affected by direct treatment with MG132 and that the effect observed could be attributed specifically to the influence of MG132 on Src1 cells. Hence, the results show that both stable overexpression of cx43 and transient overexpression of cx43 by pharmacological manipulation are able to overcome the effects of v-Src on GJIC and thereby influence cisplatin response in neighboring cells.
Figure 4. Inhibition of v-Src kinase activity or overexpression of connexin overcomes the increased survival conferred on neighboring cells by v-Src.
(A) Western blot analysis of cx43 expression in Src1 cells, Src1 cells transfected with a vector expressing human cx43 cDNA, or Src1 cells treated with the proteasome inhibitor, MG132 (100nM for 24 h). Value below each lane represent levels of cx43 expression in each sample relative to untreated Src1 cells (normalized to tubulin). (B) Visualization of monolayer growth (in the presence of puro) by Ku80-puro+ receiver cells co-mixed with the indicated MEF-derived cell lines: WT MEFs; MEFs expressing v-Src (Src1); Src1 cells overexpressing cx43 by gene transfer (Src1+cx); and Src1 cells pre-treated with the proteasome inhibitor MG132. Puro selection restricted growth only to the Ku80-puro+ receiver cells. (C) Clonogenic survival of Ku80-puro+ receiver cells co-mixed with the indicated sender cell line and treated with cisplatin in the presence or absence of the Src tyrosine kinase inhibitor PP2, as indicated. Mixed cell populations were seeded at high density, treated with PP2, then exposed to cisplatin. Following treatment, cisplatin and PP2 were removed, cells were washed with PBS, trypsinized and reseeded at low density in the presence of puro to specifically detect growth of Ku80-puro+ receiver cell colonies.
To further link src kinase activity in Src1 cells with the altered cisplatin survival of co-mixed Ku80-puro+ cells, we compared cisplatin survival of Ku80-puro+ cells in high density mixed populations of Src1 and Ku80-puro+ with or without pre-treatment with the src kinase inhibitor, PP2. Survival of Ku80-puro+ cells in this experiment was quantified by colony formation (Figure 4C), revealing that PP2 completely blocks the trans-acting pro-survival effect of v-Src on Ku80-puro+ receiver cells. This further supports our model in which v-Src kinase mediates connexin phosphorylation and reduces GJIC, thereby attenuating the transmission of the cell-to-cell death signal. Note that PP2 has no effect on the survival of the Ku80-puro+ receiver cells when they are mixed with wt cells that do not contain activated src, suggesting that malignant tissue containing cells with activated src would be sensitized to cisplatin by src kinase inhibition but that healthy tissue would be unaffected.
Discussion
We have shown here that expression of v-Src in one cell population can alter the survival of neighboring cells (even those not expressing it) to the chemotherapeutic drug, cisplatin. Stable expression of v-Src in two independent clones produced increased tyrosine phosphorylation of cx43, yielded decreased GJIC, and abrogated the density dependence of cisplatin killing in cell populations. In mixed populations in which non-v-Src expressing cells were tagged by puro resistance for detection, we found that v-Src expression in one set of cells can block cell-to-cell transmission to neighboring cells of a cytotoxic signal triggered by cisplatin exposure. Inhibition of src kinase with PP2 was found to restore transmission of the cell death signal and to increase the cisplatin killing of neighboring cells. In addition, forced overexpression of cx43 by either stable transfection or by pre-treatment of v-Src expressing cells with the proteasome inhibitor, MG132, was also found to restore transmission of the cisplatin-induced death signal to neighboring cells.
It should be noted that our work does not imply that there are no cell autonomous effects of src that can cause resistance to cytotoxic agents. Indeed, we observed this ourselves in Figure 2D, where v-Src was seen to elevate the cisplatin survival of cells treated at low density compared to the low density survival of wt cells. At the low density tested in these experiments, there is minimal cell-to-cell contact and so cell survival is determined on a cell autonomous basis.
However, by comparing cisplatin responses at high versus low cell density and in mixed cell populations, our work reveals the novel finding that src expression in one cell can elevate the survival of neighboring cells to cisplatin by disrupting cell-to-cell transmission of a death signal. This linkage of src expression to cell-to-cell communication is most clearly demonstrated in the cell mixing experiments in which the Ku80-puro+ cells showed better cisplatin survival when mixed with the Src1 or Src2 cells than when mixed with wt MEFs. This increased survival in the non-src expressing Ku80-puro+ cells was only observed when the cell populations were treated at high density, where gap junctions can be formed, and not at low density, indicating that cell-to-cell contact and intercellular communication are required.
Clinically, the overexpression and activation of src is seen in many of the cancer types that are currently treated with cisplatin, including head and neck squamous cell carcinomas, non-small cell lung cancers, and ovarian carcinomas (10-12). In this regard, our finding that the src kinase inhibitor, PP2, can reverse the effect of activated src in one cell and thereby increase the cytotoxicity of cisplatin on neighboring cells suggests a novel application for src kinase inhibitors in combination with cisplatin. One src inhibitor, Dasatinib (sprycel), is already in clinical use to treat chronic myelogenous leukemia, and our work indicates that it could have a new therapeutic application in a strategy to enhance the effectiveness of cisplatin in many solid tumors.
The regulation of connexins in cancer is also an area of active interest because it has been shown that increased connexin expression and consequent elevated GJIC in certain cancer cell lines can reverse aspects of the malignant phenotype (13, 14). Here, we have shown that the level of the gap junction protein, cx43, modulates cell-to-cell cisplatin killing, and that elevated expression of cx43 by gene transfer or by upregulation via proteasome inhibition can overcome the effects of v-Src. In this regard, since proteasome inhibitors such as bortezomib are available for clinical use, our observation that MG132 can sufficiently upregulate cx43 expression to sensitize cell populations to cisplatin may also provide the basis for a new therapeutic strategy to combine proteasome inhibitors with cisplatin treatment.
Finally, the ability of src, as well as of other oncoproteins, to promote cell growth and survival in the face of cytotoxic agents is well-established (15-17). However, no prior studies considered or even suspected that src might act in trans to alter the response of non-src expressing neighboring cells. In our prior work, in which we surveyed a series of cytotoxic agents for their ability to induce cell death via gap junction transmission, we found that only cisplatin triggers this pathway (3). Hence, it is likely that the effect of activated src identified here is specific for cisplatin exposure. Nonetheless, in addition to src, there is evidence that signaling by Ha-Ras (18, 19) and by the EGF receptor (20) may also modulate GJIC, and so it is possible that these factors may therefore also influence the survival of cells to cisplatin without having to be expressed/activated in all cells in a tumor population. If so, pharmacologic targeting of these factors may provide yet another means to sensitize malignant cell populations to cisplatin.
Acknowledgements
This work was supported by the American Cancer Society, New England Division (Jo and Gus Berkes Postdoctoral Fellowship to EPR), the Anna Fuller Fund (Postdoctoral Fellowship in Molecular Oncology to EPR), and the NIH (RO1CA113344 to PMG).
Financial Support: American Cancer Society, New England Division (Jo and Gus Berkes Postdoctoral Fellowship to EPR), the Anna Fuller Fund (Postdoctoral Fellowship in Molecular Oncology to EPR), and the NIH (RO1CA113344 to PMG)
References
- 1.Engelman JA, Zejnullahu K, Mitsudomi T, et al. MET Amplification Leads to Gefitinib Resistance in Lung Cancer by Activating ERBB3 Signaling. Science. 2007;316(5827):1039–43. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 2.Gorre ME, Mohammed M, Ellwood K, et al. Clinical Resistance to STI-571 Cancer Therapy Caused by BCR-ABL Gene Mutation or Amplification. Science. 2001;293(5531):876–80. doi: 10.1126/science.1062538. [DOI] [PubMed] [Google Scholar]
- 3.Jensen R, Glazer PM. Cell-interdependent cisplatin killing by Ku/DNA-dependent protein kinase signaling transduced through gap junctions. Proc Natl Acad Sci U S A. 2004;101(16):6134–9. doi: 10.1073/pnas.0400051101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin R, Warn-Cramer BJ, Kurata WE, Lau AF. v-Src phosphorylation of connexin 43 on Tyr247 and Tyr265 disrupts gap junctional communication. J Cell Biol. 2001;154(4):815–27. doi: 10.1083/jcb.200102027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oh SY, Dupont E, Madhukar BV, et al. Characterization of gap junctional communication-deficient mutants of a rat liver epithelial cell line. Eur J Cell Biol. 1993;60(2):250–5. [PubMed] [Google Scholar]
- 6.Czyz J, Irmer U, Schulz G, Mindermann A, Hulser DF. Gap-junctional coupling measured by flow cytometry. Exp Cell Res. 2000;255(1):40–6. doi: 10.1006/excr.1999.4760. [DOI] [PubMed] [Google Scholar]
- 7.Peterson-Roth E, Reynolds M, Quievryn G, Zhitkovich A. Mismatch repair proteins are activators of toxic responses to chromium-DNA damage. Mol Cell Biol. 2005;25(9):3596–607. doi: 10.1128/MCB.25.9.3596-3607.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gibson SL, Bindra RS, Glazer PM. CHK2-dependent phosphorylation of BRCA1 in hypoxia. Radiat Res. 2006;166(4):646–51. doi: 10.1667/RR0660.1. [DOI] [PubMed] [Google Scholar]
- 9.Musil LS, Le AC, VanSlyke JK, Roberts LM. Regulation of connexin degradation as a mechanism to increase gap junction assembly and function. J Biol Chem. 2000;275(33):25207–15. doi: 10.1074/jbc.275.33.25207. [DOI] [PubMed] [Google Scholar]
- 10.Wiener JR, Windham TC, Estrella VC, et al. Activated SRC protein tyrosine kinase is overexpressed in late-stage human ovarian cancers. Gynecol Oncol. 2003;88(1):73–9. doi: 10.1006/gyno.2002.6851. [DOI] [PubMed] [Google Scholar]
- 11.Bu R, Purushotham K, Kerr M, et al. Alterations in the level of phosphotyrosine signal transduction constituents in human parotid tumors. Proc Soc Exp Biol Med. 1996;211(3):257–64. doi: 10.3181/00379727-211-43969. [DOI] [PubMed] [Google Scholar]
- 12.Mazurenko NN, Kogan EA, Zborovskaya IB, Kisseljov FL. Expression of pp60c-src in human small cell and non-small cell lung carcinomas. Eur J Cancer. 1992;28(2-3):372–7. doi: 10.1016/s0959-8049(05)80056-5. [DOI] [PubMed] [Google Scholar]
- 13.Huang RP, Fan Y, Hossain MZ, Peng A, Zeng ZL, Boynton AL. Reversion of the neoplastic phenotype of human glioblastoma cells by connexin 43 (cx43). Cancer Res. 1998;58(22):5089–96. [PubMed] [Google Scholar]
- 14.King TJ, Fukushima LH, Hieber AD, Shimabukuro KA, Sakr WA, Bertram JS. Reduced levels of connexin43 in cervical dysplasia: inducible expression in a cervical carcinoma cell line decreases neoplastic potential with implications for tumor progression. Carcinogenesis. 2000;21(6):1097–109. [PubMed] [Google Scholar]
- 15.Masumoto N, Nakano S, Fujishima H, Kohno K, Niho Y. v-src induces cisplatin resistance by increasing the repair of cisplatin-DNA interstrand cross-links in human gallbladder adenocarcinoma cells. Int J Cancer. 1999;80(5):731–7. doi: 10.1002/(sici)1097-0215(19990301)80:5<731::aid-ijc17>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 16.Pengetnze Y, Steed M, Roby KF, Terranova PF, Taylor CC. Src tyrosine kinase promotes survival and resistance to chemotherapeutics in a mouse ovarian cancer cell line. Biochem Biophys Res Commun. 2003;309(2):377–83. doi: 10.1016/j.bbrc.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 17.Qin B, Ariyama H, Baba E, et al. Activated Src and Ras induce gefitinib resistance by activation of signaling pathways downstream of epidermal growth factor receptor in human gallbladder adenocarcinoma cells. Cancer Chemother Pharmacol. 2006;58(5):577–84. doi: 10.1007/s00280-006-0219-4. [DOI] [PubMed] [Google Scholar]
- 18.Lee KW, Jung JW, Kang KS, Lee HJ. p38 is a key signaling molecule for H-ras-induced inhibition of gap junction intercellular communication in rat liver epithelial cells. Ann N Y Acad Sci. 2004;1030:258–63. doi: 10.1196/annals.1329.032. [DOI] [PubMed] [Google Scholar]
- 19.de Feijter AW, Trosko JE, Krizman DB, Lebovitz RM, Lieberman MW. Correlation of increased levels of Ha-ras T24 protein with extent of loss of gap junction function in rat liver epithelial cells. Mol Carcinog. 1992;5(3):205–12. doi: 10.1002/mc.2940050307. [DOI] [PubMed] [Google Scholar]
- 20.Lau AF, Kanemitsu MY, Kurata WE, Danesh S, Boynton AL. Epidermal growth factor disrupts gap-junctional communication and induces phosphorylation of connexin43 on serine. Mol Biol Cell. 1992;3(8):865–74. doi: 10.1091/mbc.3.8.865. [DOI] [PMC free article] [PubMed] [Google Scholar]