Figure 2.
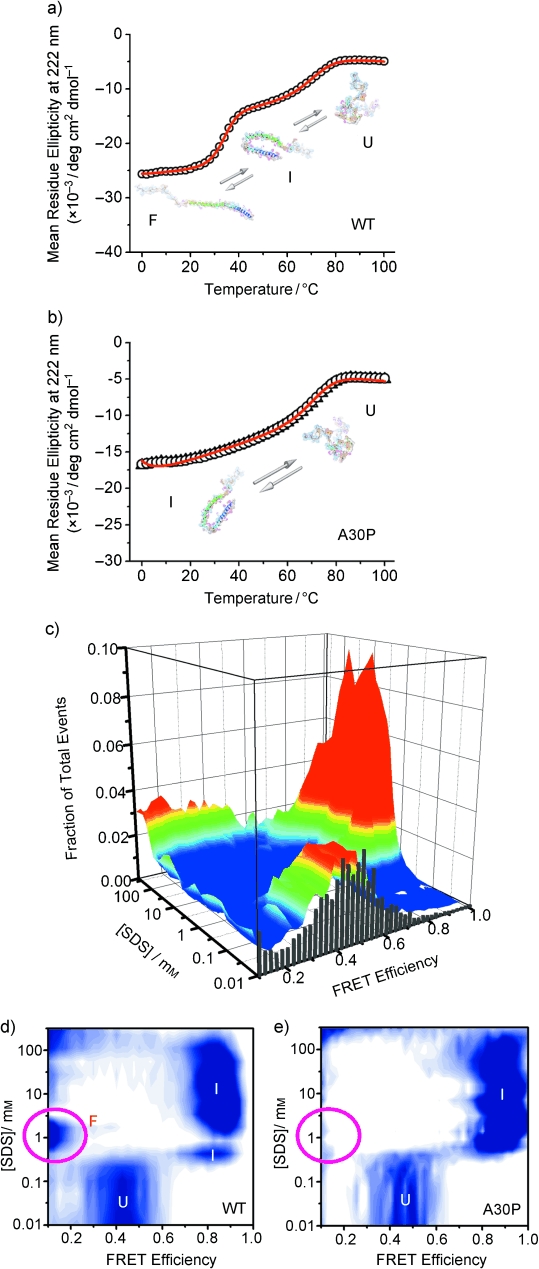
Single-molecule and ensemble characterization of the effect of the PD-linked A30P mutation on the coupled binding and folding of α-synuclein. a,b) Thermal unfolding of WT α-synuclein (a) and the A30P mutant (b) bound to SDS monomers, as monitored at the ensemble level by CD spectroscopy in the presence of SDS (1 mm). c) Conformational transitions of A30P α-synuclein induced by changes in ligand concentration at room temperature, as detected at the single-molecule level by smFRET. d) Two-dimensional contour plot of previously reported WT data16 for comparison with the corresponding plot for the A30P mutant in (e). e) Two-dimensional contour plot of the raw data presented in (c). U, I, and F are different protein conformational states that exhibit characteristic FRET efficiency16 and helicity.10 U is the natively unfolded state, I corresponds to a bent helical structure previously solved by NMR spectroscopy (1XQ8),18 and F is an extended α-helix structure. (See the Supporting Information for additional details).
