Table 2a.
Affinities (Ki=nM) of 3-substituted β-carbolines at αxβ3γ2(x=1–3,5,6) receptor subtypes
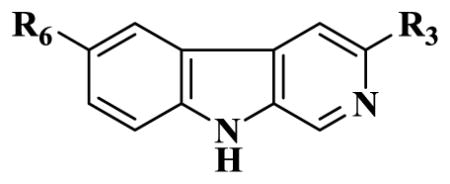 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Ligands | R6 | R3 | α1 | α2 | α3 | α4 | α5 | α6 |
| BCCE | H | CO2Et | 1.2 | 4.9 | 5.7 | 1000 | 26.8 | 2700 |
| 15 | H | CO2CH2CF3 | 3.0 | 24.5 | 41.7 | >500 | 125.7 | >2000 |
| 16(WYB09-1) | H | CO2CH(CF3)2 | 3.99 | 8 | 32 | 1000 | 461 | 2000 |
| 17(WYB23-1) | H | CO2CH2CCl3 | 10 | 33 | 43 | 1000 | 189 | 2000 |
| 18(WYB17) | H | CO2CH(CH3)CCl3 | 2000 | 2000 | 2000 | 3000 | 2000 | 5000 |
| 19(CMA64) | H | CO2CH(CH3)C2H5 | 18 | 60 | 116 | NA | 216 | >2000 |
| 20(CMA69) | H | CO2CH(CF3)C2H5 | 1000 | 1000 | 1000 | NA | 1000 | >2000 |
| 25(WY-B-24) | 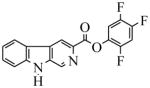 |
22.0 | 177 | 44.8 | 3000 | 422 | 3000 | |
| 26(CM-A-77) | 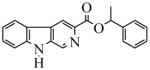 |
33.5 | 1000 | 1000 | 1000 | 1000 | 3000 | |
The affinity of compounds at GABAA/BzR recombinant subtypes was measured by competition for [3H]flunitrazepam binding to HEK cell membranes expressing human receptors of composition α1β3γ2, α2β3γ2, α3β3γ2, α4β3γ2, α5β3γ2 and α6β3γ2.119 Data represent the average of at least three determinations with a SEM of ±5%.
