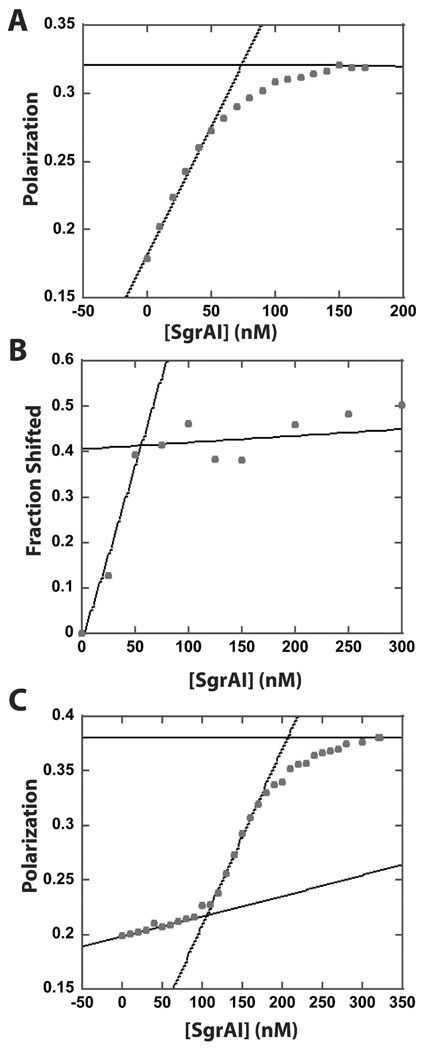
Figure 2.
Determination of the stoichiometry of binding of SgrAI to 18-2 and 18-1. A. Titration of 75 nM HEX labeled 18-2 in Buffer 2 (Table 1) at 4°C. The intercept of the two lines is 69 nM SgrAI dimer, indicating a 1:1 stoichiometry of SgrAI dimer to 18-2 duplex with 92% activity of SgrAI enzyme. B. Native PAGE gel shift of 100 nM 32P labeled 18-1 (of which 40% is double stranded). The intercept occurs at 55 nM SgrAI, suggesting 1:1 binding of 18-1 to SgrAI dimer with 73% enzyme activity. C. Titration of 100 nM HEX labeled 18-1 in Buffer 2 (Table 1) at 4°C. The first intercept at 109 nM SgrAI dimer indicates a 1:1 stoichiometry of SgrAI dimer to 18-1 duplex with 92% activity of SgrAI enzyme, the second intercept at 207 nM SgrAI dimer indicates a second SgrAI dimer binds the original 1:1 complex.