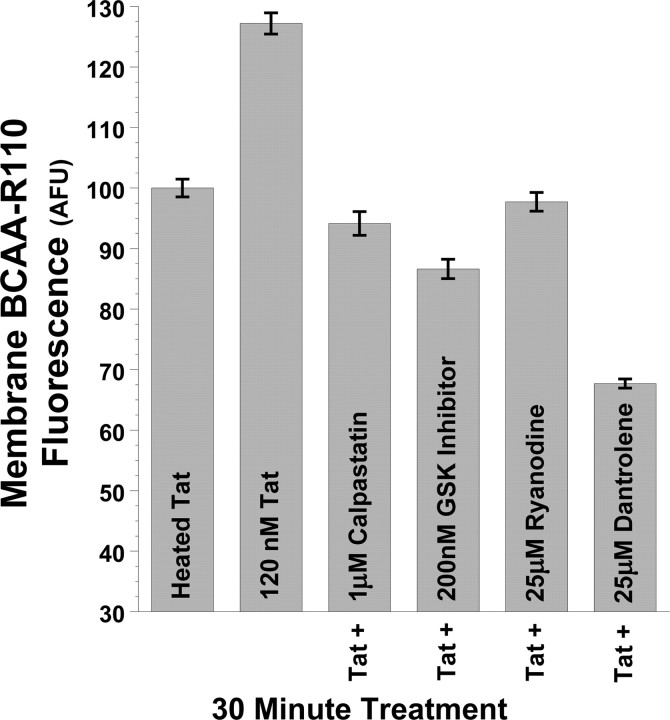
Figure 5.
Tat increases calpain protease activity at the plasma membrane via RyR and GSK-3β pathways. Tat also increased membrane-proximal calpain protease activity in PC12 cells, as shown by TIRFM imaging of the cell-permeable fluorescent calpain substrate BCAA-R110. This substrate is nonfluorescent until cleaved by proteases at the amide bond, thus releasing the rhodamine 110 fluorophore and rendering it available for excitation. Imaging this dye by TIRFM, under the same treatment conditions as in Figure 4, we show that 30 min treatment of PC12 cells again with 120 nm Tat increases calpain protease activity at the plasma membrane versus control cells treated with 120 nm heat-inactivated Tat. Just as 25 μm dantrolene, 25 μm ryanodine, 1 μm calpastatin peptide, and 200 nm N-(4-methoxybenzyl)-N′-(5-nitro-1,3-thiazol-2-yl)urea prevent 120 nm Tat-induced increases in plasma membrane DAT (Fig. 4), these treatments also prevent Tat-induced increases in membrane calpain activity (Fig. 5). Together, these results suggest membrane-localized control of DAT trafficking by RyR- and GSK-3β-mediated activation of membrane-proximal calpain proteases. After 30 min treatment with 120 nm Tat, mean fluorescent BCAA-R110 signal intensity at the membrane was quantified from the cell membrane surface area of ≥30 cells collated over multiple experimental treatment runs and imaged under TIRFM, and then averaged and expressed as raw intensity (arbitrary fluorescent units) ± SEM.