Figure 6.
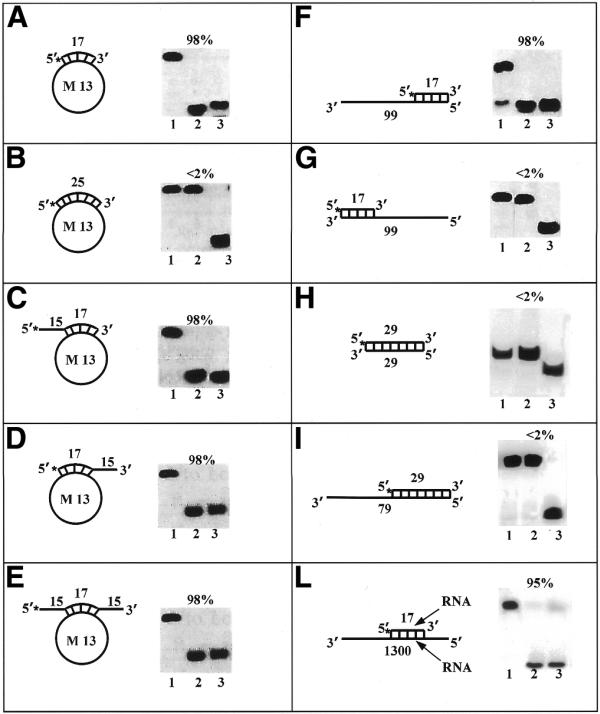
Helicase activity with various substrates. Each panel shows the structure of the substrate used and the autoradiogram of the gel. Asterisks denote the 32P-labeled end. Percent unwinding is shown above each autoradiogram. Lanes 1 and 3 correspond to control reactions without enzyme and heat-denatured substrate, respectively. Lane 2 corresponds to the reaction with pure HDH V (∼0.4 nM). Direction of unwinding is analyzed in (F) and (G). Substrate H corresponds to the double strand FUSE obtained by annealing the FUSE coding and NCS oligonucleotides. Substrate I corresponds to substrate H with the addition of a 50 pT tail at the 3′ end of the coding strand oligonucleotide.
