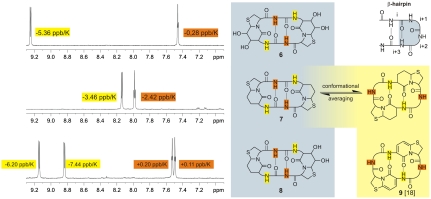
Fig. 2.
1H NMR (600 MHz, DMSO-d6). The amide region from the 1H NMR (upper left) of cyclo[Hot═Tap-Gly-]2 (6) identifies a solvent-exposed amide proton at 9.25 ppm (yellow) exhibiting a strong temperature dependence (-5.36 ppb/K). The triplet at 7.45 ppm (Gly-NH, brown) on the contrary is hardly influenced by the temperature (-0.28 ppb/K) as expected for a proton engaged in an intramolecular hydrogen bond. Based on these data, Hot═Tap is expected to occupy the turn position in hexapeptide 6 (gray background, top). The amide region from the 1H NMR (middle left) of cyclo[BTD-Gly-]2 (7) shows averaged chemical shifts and temperature gradients caused by the conformational adaptability of BTD, which fits at least two different positions in the hexapeptide. The lower left 1H NMR of the mixed hexapeptide cyclo[Hot═Tap-Gly-BTD-Gly-]2 (8) is again similar to the 1H NMR of 6 with a doubled signal caused by the broken C2 symmetry. The proposed alternative conformation of 7 is shown with a yellow background similar to 9 (18) as an example of a cyclic hexapeptide populating an alternative conformation (yellow background).