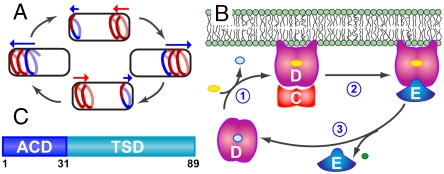
Fig. 1.
(A) Model of Min protein oscillation in rod-shaped bacteria. MinD forms a coiled array (red) that extends from the cell pole and is capped by a MinE-rich region known as the E ring (blue). Disassembly of the array toward the cell pole (in the direction of the blue arrow) is accompanied by assembly of a new MinD coil at the opposing pole (red arrow). (B) Schematic diagram of the Min cycle. (1) MinD (magenta) bound to ADP (light blue) undergoes nucleotide exchange with ATP (yellow), to give rise to a membrane-bound state that can bind MinC (red), the inhibitor of cell division septum formation. (2) MinE (blue) binding to MinD displaces MinC and (3) stimulates ATP hydrolysis by MinD to release inorganic phosphate (green). MinE is released from the complex while ADP-bound MinD also dissociates from the membrane, allowing the cycle to begin again. (C) Functional domain structure of MinE. Residues 1–30 comprise the anti-MinCD (ACD) domain while the remaining C-terminal residues contain the TSD.