Abstract
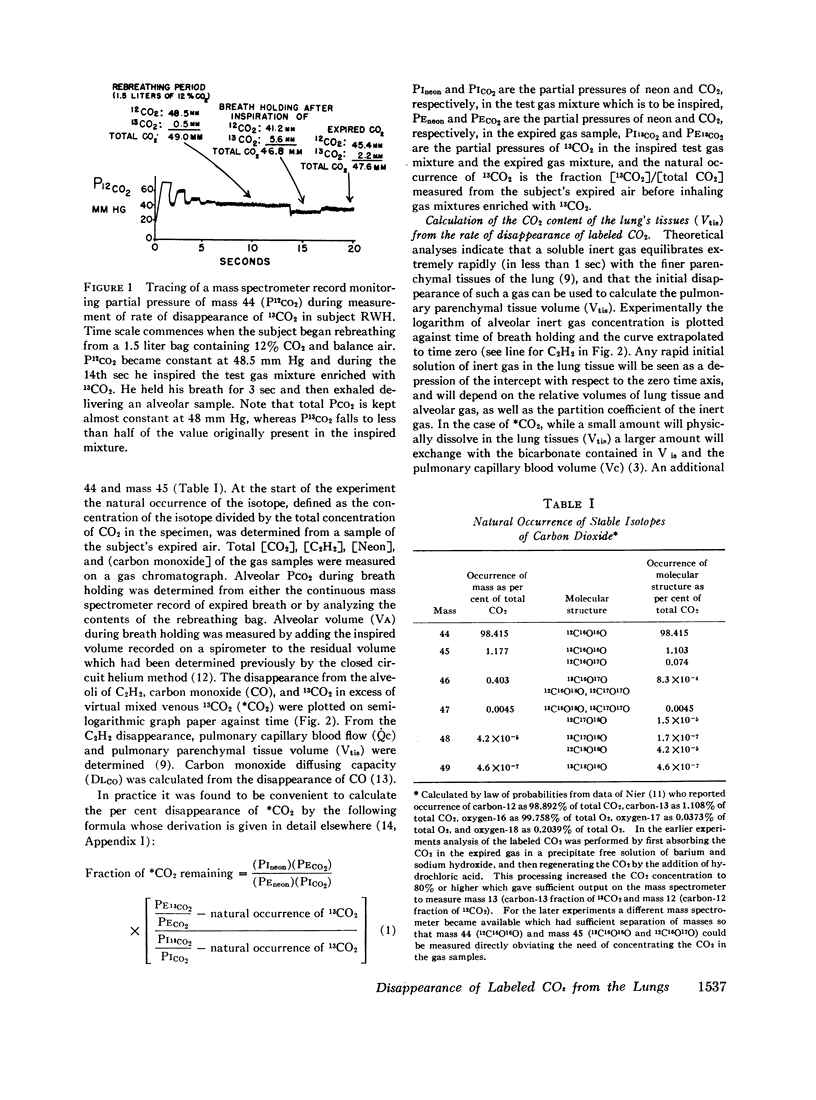
The dynamics of CO2 exchange in the lungs of man was studied by observing the rate of disappearance of a stable isotope of CO2 (13CO2) from the alveolar gas during breath holding. Over 50% of the inspired isotope disappeared within the first 3 sec followed by a moderately rapid logarithmic decline in which one-half of the remaining 13CO2 disappeared every 10 sec.
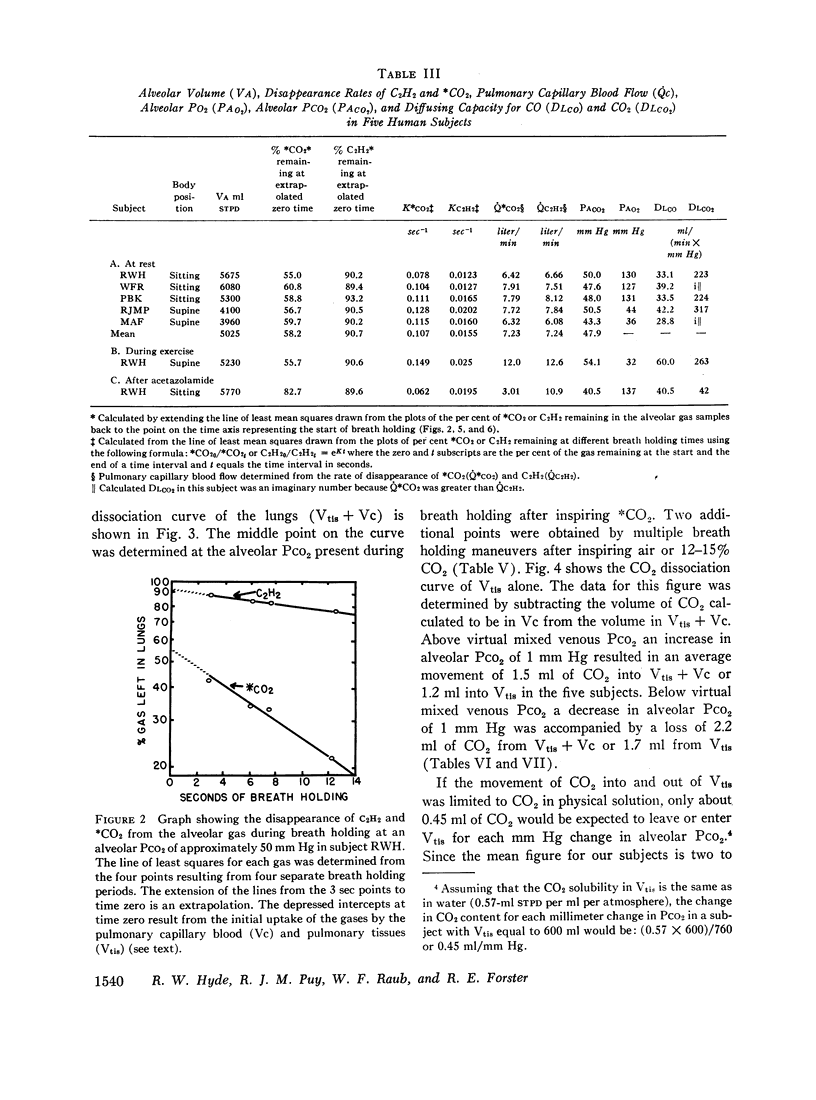
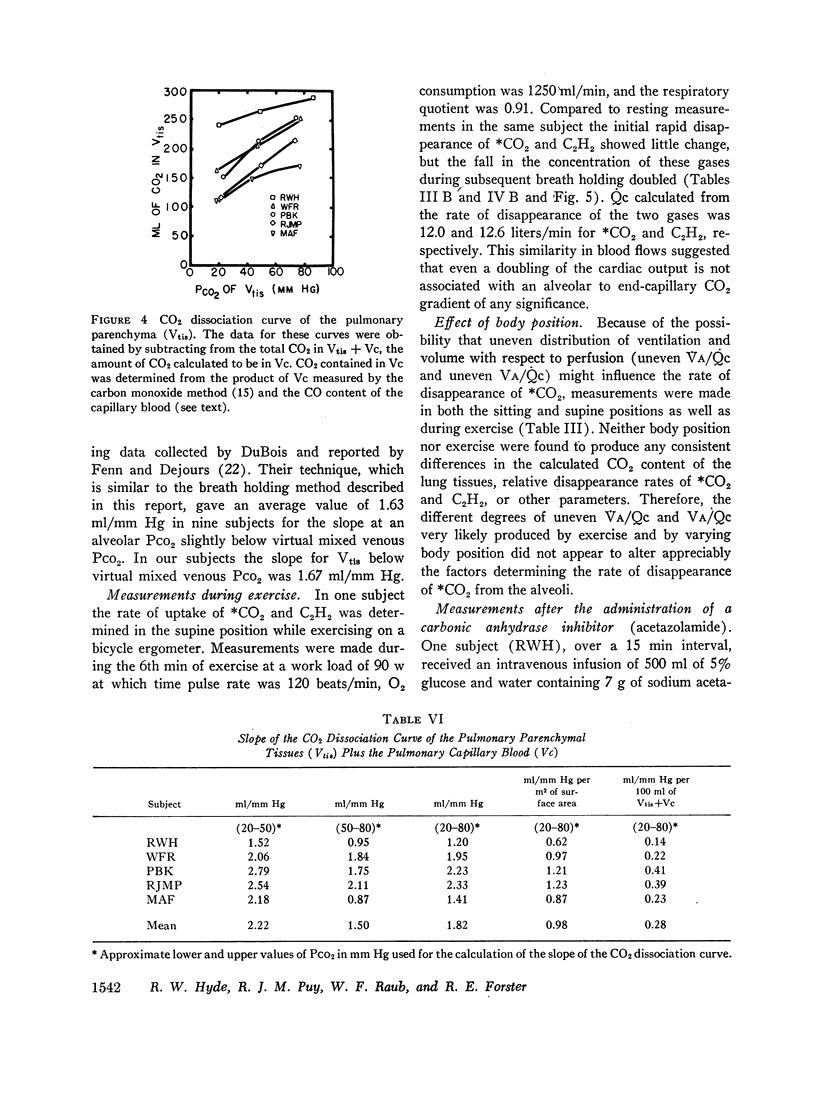
The large initial disappearance of 13CO2 indicated that alveolar 13CO2 equilibrated in less than 3 sec with the CO2 stored in the pulmonary tissues and capillary blood. The volume of CO2 in the pulmonary tissues calculated from this initial disappearance was 200 ml or 0.33 ml of CO2 per milliliter of pulmonary tissue volume.
The alveolar to end-capillary gradient for 13CO2 was calculated by comparing the simultaneous disappearance rates of 13CO2 and acetylene. At rest and during exercise this gradient for 13CO2 was either very small or not discernible, and diffusing capacity for CO2 (DLCO2) exceeded 200 ml/(min × mm Hg).
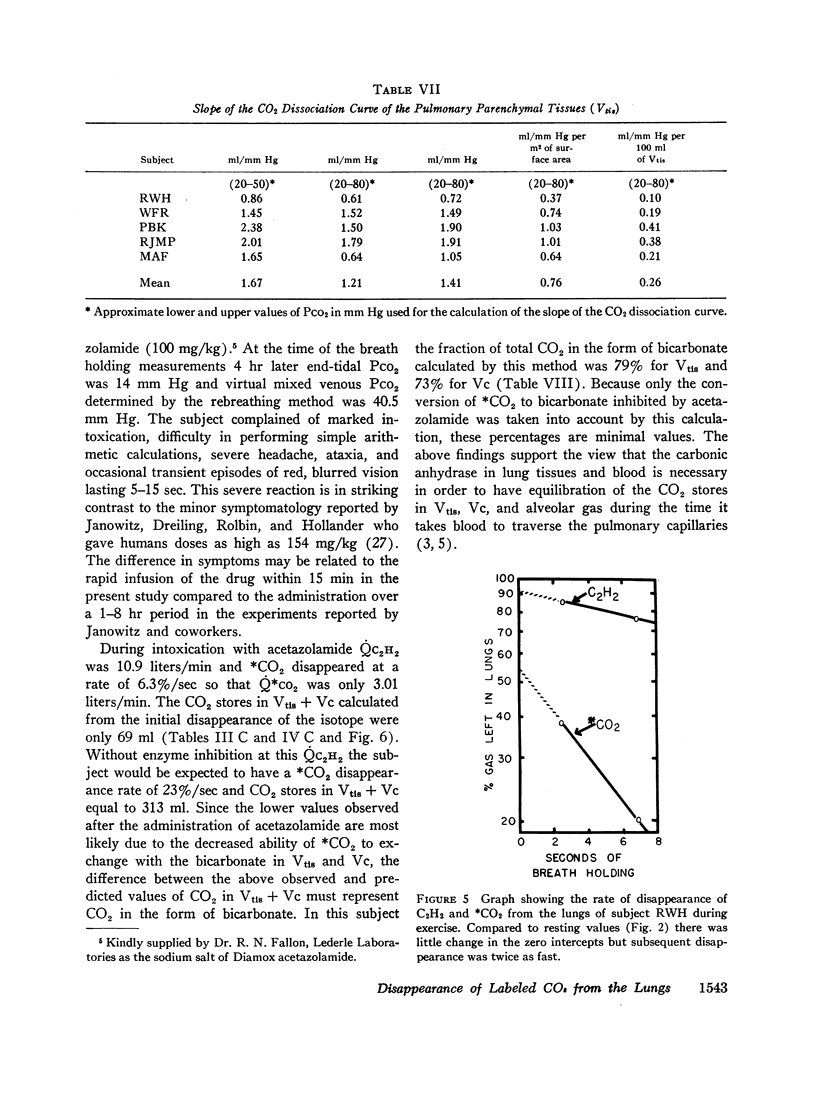
After the administration of a carbonic anhydrase inhibitor the rate of disappearance of 13CO2 decreased markedly. DLCO2 fell to 42 ml/(min × mm Hg) and at least 70% of the exchange of 13CO2 with the CO2 stores in the pulmonary tissues and blood was blocked by the inhibitor. These changes were attributed to impairment of exchange of 13CO2 with the bicarbonate in the pulmonary tissues and blood.
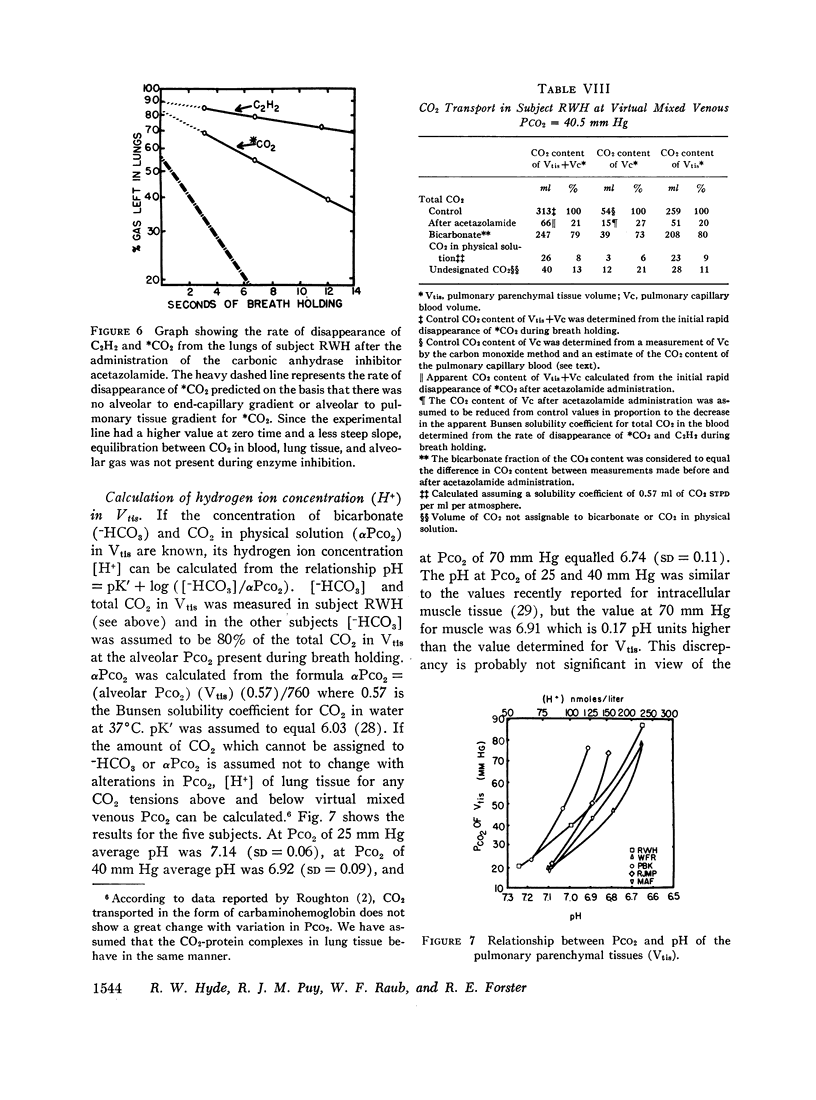
The pH of the pulmonary tissues (Vtis) was determined by a method based on the premise that the CO2 space in the pulmonary tissues blocked by the inhibitor represented total bicarbonate content. At an alveolar PCO2 of 40 mm Hg pH of Vtis equalled 6.97 ± 0.09.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADLER S., ROY A., RELMAN A. S. INTRACELLULAR ACID-BASE REGULATION. I. THE RESPONSE OF MUSCLE CELLS TO CHANGES IN CO2 TENSION OR EXTRACELLULAR BICARBONATE CONCENTRATION. J Clin Invest. 1965 Jan;44:8–20. doi: 10.1172/JCI105129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANDERSEN O. S., ENGEL K., JORGENSEN K., ASTRUP P. A Micro method for determination of pH, carbon dioxide tension, base excess and standard bicarbonate in capillary blood. Scand J Clin Lab Invest. 1960;12:172–176. doi: 10.3109/00365516009062419. [DOI] [PubMed] [Google Scholar]
- BLAKEMORE W. S., FORSTER R. E., MORTON J. W., OGILVIE C. M. A standardized breath holding technique for the clinical measurement of the diffusing capacity of the lung for carbon monoxide. J Clin Invest. 1957 Jan;36(1 Pt 1):1–17. doi: 10.1172/JCI103402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOSMAN A. R., LEE G. D., MARSHALL R. THE EFFECT OF PULSATILE CAPILLARY BLOOD FLOW UPON GAS EXCHANGE WITHIN THE LUNGS OF MAN. Clin Sci. 1965 Apr;28:295–309. [PubMed] [Google Scholar]
- CAIN S. M., OTIS A. B. Carbon dioxide transport in anesthetized dogs during inhibition of carbonic anhydrase. J Appl Physiol. 1961 Nov;16:1023–1028. doi: 10.1152/jappl.1961.16.6.1023. [DOI] [PubMed] [Google Scholar]
- CARTER E. T., CLARK R. T., Jr Effects of carbonic anhydrase inhibition during acute hypoxia. J Appl Physiol. 1958 Jul;13(1):47–52. doi: 10.1152/jappl.1958.13.1.47. [DOI] [PubMed] [Google Scholar]
- CHINARD F. P., ENNS T., NOLAN M. F. Contributions of bicarbonate ion and of dissolved CO2 to expired CO2 in dogs. Am J Physiol. 1960 Jan;198:78–88. doi: 10.1152/ajplegacy.1960.198.1.78. [DOI] [PubMed] [Google Scholar]
- CONSTANTINE H. P., CRAW M. R., FORSTER R. E. RATE OF THE REACTION OF CARBON DIOXIDE WITH HUMAN RED BLOOD CELLS. Am J Physiol. 1965 Apr;208:801–811. doi: 10.1152/ajplegacy.1965.208.4.801. [DOI] [PubMed] [Google Scholar]
- Carter N. W., Rector F. C., Jr, Campion D. S., Seldin D. W. Measurement of intracellular pH of skeletal muscle with pH-sensitive glass microelectrodes. J Clin Invest. 1967 Jun;46(6):920–933. doi: 10.1172/JCI105598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway E. J., Fearon P. J. The acid-labile CO(2) in mammalian muscle and the pH of the muscle fibre. J Physiol. 1944 Dec 15;103(3):274–289. doi: 10.1113/jphysiol.1944.sp004076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DORRIS R., OLIVIA J. V., RODMAN T. DICHLORPHENAMIDE, A POTENT CARBONIC ANHYDRASE INHIBITOR. EFFECT ON ALVEOLAR VENTILATION, VENTILATION-PERFUSION RELATIONSHIPS AND DIFFUSION IN PATIENTS WITH CHRONIC LUNG DISEASE. Am J Med. 1964 Jan;36:79–86. doi: 10.1016/0002-9343(64)90150-0. [DOI] [PubMed] [Google Scholar]
- DUBOIS A. B. Alveolar CO2 and O2 during breath holding, expiration, and inspiration. J Appl Physiol. 1952 Jul;5(1):1–12. doi: 10.1152/jappl.1952.5.1.1. [DOI] [PubMed] [Google Scholar]
- DUBOIS A. B., BRITT A. G., FENN W. O. Alveolar CO2 during the respiratory cycle. J Appl Physiol. 1952 Jan;4(7):535–548. doi: 10.1152/jappl.1952.4.7.535. [DOI] [PubMed] [Google Scholar]
- DUBOIS A. B., FENN W. O., BRITT A. G. CO2 dissociation curve of lung tissue. J Appl Physiol. 1952 Jul;5(1):13–16. doi: 10.1152/jappl.1952.5.1.13. [DOI] [PubMed] [Google Scholar]
- Enns T. Facilitation by carbonic anhydrase of carbon dioxide transport. Science. 1967 Jan 6;155(3758):44–47. doi: 10.1126/science.155.3758.44. [DOI] [PubMed] [Google Scholar]
- FEISAL K. A., SACKNER M. A., DUBOIS A. B. Comparison between the time available and the time required for CO2 equilibration in the lung. J Clin Invest. 1963 Jan;42:24–28. doi: 10.1172/JCI104692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FENN W. O., DEJOURS P. Composition of alveolar air during breath holding with and without prior inhalation of oxygen and carbon dioxide. J Appl Physiol. 1954 Nov;7(3):313–319. doi: 10.1152/jappl.1954.7.3.313. [DOI] [PubMed] [Google Scholar]
- HACKNEY J. D., SEARS C. H., COLLIER C. R. Estimation of arterial CO2 tension by rebreathing technique. J Appl Physiol. 1958 May;12(3):425–430. doi: 10.1152/jappl.1958.12.3.425. [DOI] [PubMed] [Google Scholar]
- Hyde R. W., Forster R. E., Power G. G., Nairn J., Rynes R. Measurement of O2 diffusing capacity of the lungs with a stable O2 isotope. J Clin Invest. 1966 Jul;45(7):1178–1193. doi: 10.1172/JCI105424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde R. W., Rynes R., Power G. G., Nairn J. Determination of distribution of diffusing capacity in relation to blood flow in the human lung. J Clin Invest. 1967 Mar;46(3):463–474. doi: 10.1172/JCI105548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JANOWITZ H. D., DREILING D. A., ROLBIN H. L., HOLLANDER F. Inhibition of the formation of hydrochloric acid in the human stomach by diamox; the role of carbonic anhydrase in gastric secretion. Gastroenterology. 1957 Sep;33(3):378–384. [PubMed] [Google Scholar]
- Jones N. L., Campbell E. J., McHardy G. J., Higgs B. E., Clode M. The estimation of carbon dioxide pressure of mixed venous blood during exercise. Clin Sci. 1967 Apr;32(2):311–327. [PubMed] [Google Scholar]
- MCNICOL M. W., PRIDE N. B. Dichlorphenamide in chronic respiratory failure. Lancet. 1961 Apr 29;1(7183):906–908. doi: 10.1016/s0140-6736(61)91770-6. [DOI] [PubMed] [Google Scholar]
- MITHOEFER J. C., DAVIS J. S. Inhibition of carbonic anhydrase: effect on tissue gas tensions in the rat. Proc Soc Exp Biol Med. 1958 Aug-Sep;98(4):797–801. doi: 10.3181/00379727-98-24189. [DOI] [PubMed] [Google Scholar]
- MITHOEFER J. C. Inhibition of carbonic anhydrase: its effect on carbon dioxide elimination by the lungs. J Appl Physiol. 1959 Jan;14(1):109–115. doi: 10.1152/jappl.1959.14.1.109. [DOI] [PubMed] [Google Scholar]
- NAIMARK A., BRODOVSKY D. M., CHERNIACK R. M. The effect of a new carbonic anhydrase inhibitor (dichlorphenamide) in respiratory insufficiency. Am J Med. 1960 Mar;28:368–375. doi: 10.1016/0002-9343(60)90168-6. [DOI] [PubMed] [Google Scholar]
- ROUGHTON F. J., FORSTER R. E. Relative importance of diffusion and chemical reaction rates in determining rate of exchange of gases in the human lung, with special reference to true diffusing capacity of pulmonary membrane and volume of blood in the lung capillaries. J Appl Physiol. 1957 Sep;11(2):290–302. doi: 10.1152/jappl.1957.11.2.290. [DOI] [PubMed] [Google Scholar]
- SACKNER M. A., FEISAL K. A., DUBOIS A. B. DETERMINATION OF TISSUE VOLUME AND CARBON DIOXIDE DISSOCIATION SLOPE OF THE LUNGS IN MAN. J Appl Physiol. 1964 May;19:374–380. doi: 10.1152/jappl.1964.19.3.374. [DOI] [PubMed] [Google Scholar]
- SACKNER M. A., FEISAL K. A., KARSCH D. N. SIZE OF GAS EXCHANGE VESSELS IN THE LUNG. J Clin Invest. 1964 Sep;43:1847–1855. doi: 10.1172/JCI105058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SONI J., FEISAL K. A., DUBOIS A. B. The rate of intrapulmonary blood gas exchange in living animals. J Clin Invest. 1963 Jan;42:16–23. doi: 10.1172/JCI104691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STROMME J. H., FOG J. Effect of acetazolamide on respiratory gas exchange during hyperventilation in man. J Appl Physiol. 1962 Jan;17:6–8. doi: 10.1152/jappl.1962.17.1.6. [DOI] [PubMed] [Google Scholar]
- WEST J. B., DOLLERY C. T. Distribution of blood flow and ventilation-perfusion ratio in the lung, measured with radioactive carbon dioxide. J Appl Physiol. 1960 May;15:405–410. doi: 10.1152/jappl.1960.15.3.405. [DOI] [PubMed] [Google Scholar]
- Warburg E. J. Studies on Carbonic Acid Compounds and Hydrogen Ion Activities in Blood and Salt Solutions. A Contribution to the Theory of the Equation of Lawrence J. Henderson and K. A. Hasselbach: Introduction. Biochem J. 1922;16(2):153–154. doi: 10.1042/bj0160153. [DOI] [PMC free article] [PubMed] [Google Scholar]


