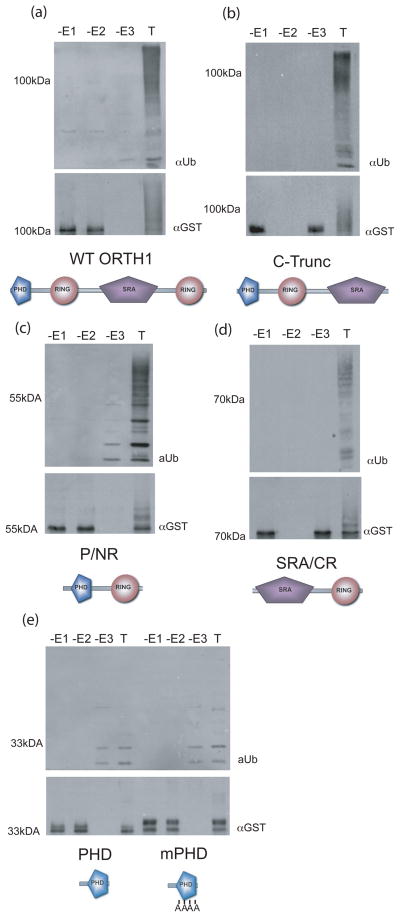
Figure 4. ORTH1 proteins with either RING domain, but not the PHD, have E3 ligase activity.
Truncations of ORTH1 (name and schematic form under their respective western blots) were expressed as GST fusions and tested for in vitro ubiquitylation activity.
(a-e) Top, anti-ubiquitin immunoblot to detect ubiquitylated products; bottom, anti-GST to visualize E3 ligase. Reactions lacking either E1, E2 or E3 (-E1, -E2, -E3, respectively) are compared to comparable reaction with all components (T).
(e) mPHD has the CCHC of the core metal ligands, positions 3-6, all changed to alanine. Full length ORTH1 assays (a) were included for comparison.