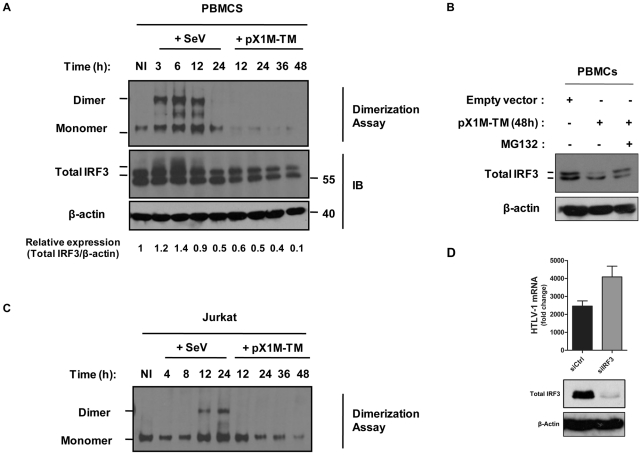
Figure 6. HTLV-1 induces degradation of endogenous IRF3.
(A). PBMCs from healthy individuals were electroporated with either HTLV-1 provirus (pX1M-TM) or empty vector. PBMCs transfected with the control vector were subsequently infected with SeV. At the indicated times, lysates from transfected or infected PBMCs were electrophoretically resolved under non-denaturing or denaturing conditions. Western blot analysis was used to locate monomer and dimer forms of IRF3 in the non-denaturing gel (upper panel), and global IRF3 protein in the denaturing gel (lower panel). Immunoblotting against β-actin was used as a loading control. Total IRF3 protein expression levels (upper band) were quantified and normalized to β-actin levels using the Scion Image 4.0 software program. (B) Degradation of IRF3 is inhibited by the proteasome inhibitor (MG132). PBMCs were treated as indicated in (A). At 48 h post-HTLV-1 provirus, cells were incubated with 5 µM of MG132 for 6 h. Cells lysates were prepared and equal amounts of protein (20 µg) were resolved by SDS-PAGE followed by immunoblotting against IRF3, with β-actin shown as a loading control. (C) Same experiments and analysis for IRF3 dimerization were performed in the Jurkat leukemic T cell line. (D) Loss of IRF3 enhances HTLV-1 mRNA load in infected T cells. Jurkat cells were electroporated with control or a pool of IRF3 specific-siRNAs, and re-transfected at 24 h with HTLV-1 provirus (pX1M-TM) vector. At 72 h post-transfection, total RNA was extracted and analyzed for HTLV-1 mRNA levels. Cells lysates were prepared at 72 h post-electroporation, and equal amounts of protein (20 µg) were resolved by SDS-PAGE followed by immunoblotting against IRF3, with β-actin shown as a loading control.