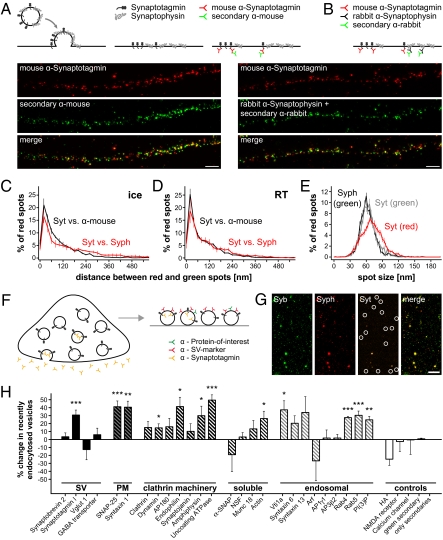
Fig. 4.
Maintenance of the molecular identity of the vesicle during recycling. (A–E) Synaptotagmin and synaptophysin remain largely coclustered on exocytosis. (A) Colocalization positive control. Only recently exocytosed vesicles are investigated, with the experiments performed after blocking the surface synaptotagmin epitopes with nonfluorescent antibodies (not shown in the cartoon; Fig. S7A). Neurons are then incubated with Atto647N-coupled mouse anti-synaptotagmin antibodies (Atto-tec; red). Endocytosis is blocked either by keeping the neurons on ice or by incubating them at room temperature in absence of divalents (Fig. S7B) to prevent the internalization of the label. After fixation, the samples are immunostained with secondary anti-mouse antibodies coupled to Atto590 (Atto-tec; green). Note the high colocalization in two-color STED microscopy. (Scale bar: 1 μm.) (B) Synaptotagmin/synaptophysin colocalization. Neurons are incubated with Atto647N-coupled mouse anti-synaptotagmin antibodies (red) and rabbit antibodies that recognize the luminal domain of synaptophysin. After fixation, the samples are immunostained with secondary anti-rabbit antibodies (Atto590; green) to visualize the synaptophysin staining. (Scale bar: 1 μm.) Spot-to-spot distance analysis is performed for experiments in which endocytosis was blocked by low temperature on ice (C) or at room temperature in the absence of divalents (D). Note that the two sets of distributions are similar [although Kolmogorov Smirnov tests detect a significant difference for C (P = 0.0057)]. (E) Spot size. Both synaptotagmin and synaptophysin form well-defined clusters of about 60–70 nm, within the resolution of the microscope. (C–E) All data represent the mean ± SEM from 3 to 6 independent experiments. (F–H) Change of synaptic vesicle composition on endocytosis. (F) Synaptosomes were purified and stimulated by depolarization (50 mM KCl) in presence of fluorescently coupled antibodies against the luminal domain of synaptotagmin. The vesicles were isolated, adsorbed onto glass coverslips, and immunostained for a synaptic vesicle marker (red) and a protein of interest (green). (G) Typical images of vesicles immunostained for synaptobrevin (Syb) as protein of interest and synaptophysin (Syph) as a synaptic vesicle marker. Several recently endocytosed vesicles are indicated in the (dim) synaptotagmin (Syt) channel. (Scale bar: 7.5 μm.) (H) Summary of the change in protein composition for the recently endocytosed vesicles vs. general pool vesicles. Several plasma membrane, clathrin-mediated endocytosis machinery, and early endosomal proteins are enriched in the recently endocytosed vesicle pool. Asterisks indicate significant changes (*P < 0.05; **P < 0.01; ***P < 0.001; t tests). Data are presented as the mean ± SEM of 3–39 independent experiments (typically 3–6 experiments; data for HA show the mean ± range of values for 2 independent experiments, data for “only secondaries” represent 1 experiment). A complete description and discussion are presented in Fig. S8.