Abstract
The marginal zone is a cellular niche bordering the marginal sinus of the spleen that contains specialized B-cell and macrophage subsets poised to capture bloodborne antigens. Marginal zone B cells are retained in this niche by integrin-mediated signaling induced by G protein-coupled receptors (GPCRs) and, likely, the B-cell receptor (BCR). Sphingosine-1-phosphate (S1P) signaling via the S1P family of GPCRs is known to be essential for B-cell localization in the marginal zone, but little is known about the downstream signaling events involved. Here, we demonstrate that the adaptor protein SHEP1 is required for marginal zone B-cell maturation. SHEP1 functions in concert with the scaffolding protein CasL, because we show that SHEP1 and CasL are constitutively associated in B cells. SHEP1 association is required for the BCR or S1P receptor(s) to induce the conversion of CasL into its serine/threonine hyperphosphorylated form, which is important for lymphocyte adhesion and motility. Thus, SHEP1 orchestrates marginal zone B-cell movement and retention as a key downstream effector of the BCR and S1P receptors.
Keywords: Cas-Hef1–associated signal transducer, Sh2d3c, migration, sphingosine-1-phosphate, signaling
Adhesion and migration are required for B-cell development, differentiation, and function. B cells traffic to follicular regions in the secondary lymphoid tissues in response to chemokines produced by the follicular stroma (1). In the spleen, the marginal zone (MZ) surrounding the white pulp contains a subset of B cells that are poised to respond to antigens delivered via the blood sinuses. MZ B cells are retained in this niche by integrin signaling induced by “outside-in” signaling via G protein-coupled receptors (GPCRs) and, likely, the B-cell receptor (BCR) (2).
The lipid mediator, sphingosine-1-phosphate (S1P), has been shown to be a prominent factor in guiding B cells to the MZ niche (3, 4). Similar to chemokine receptors, such as CXCR4 and CXCR5, S1P receptors act in B cells by coupling to heterotrimeric G proteins and mobilizing calcium (5). S1P has also been shown to induce proximal phosphorylation of focal adhesion kinase and paxillin in fibroblasts, and promote the phosphorylation of downstream adaptors and activation of Ras family GTPases to affect adhesion and migration (6). MZ B cells are particularly sensitive to deletions of certain GTPases, GAPs (GTPase-activating proteins), and GEFs that promote integrin activation, cell adhesion, and cell migration. For example, deficiencies in Rap1A, Rap1B, DOCK2, or the RhoGEF Lsc result in the loss of B-cell chemotactic responses and in a marked reduction in MZ B cells (7–11). These findings are consistent with the requisite roles of LFA-1 and VLA-4 integrins, which are highly expressed on MZ B cells compared with follicular B cells (12, 13). BCR and CXCR4 signaling have also been shown to induce the tyrosine phosphorylation of the scaffolding protein CasL/HEF1 (14, 15). Tyrosine phosphorylation of CasL is required for its interaction with the adaptor CrkL, allowing CrkL to associate with C3G, a GEF for Rap1 that promotes integrin activity and DOCK2, a GEF for Rac1 that promotes cell migration (16–18). The importance of CasL in mediating integrin signaling was demonstrated by the observed loss of MZ B cells in CasL−/− mice (15).
To better understand the mechanisms of B-cell retention in the MZ, we became interested in the adaptor molecule SHEP1 (SH2 domain-containing Eph receptor-binding protein 1), also known as Sh2d3c or Cas-Hef1–associated signal transducer (CHAT), which belongs to a family of proteins that feature an N-terminal SH2 domain linked to a GEF-like domain (19). Other family members include NSP1 and BCAR3/AND34 (19). The SHEP1 gene consists of 15 exons with alternative splicing occurring among the first six exons, resulting in isoforms SHEP1α (long), SHEP1β, SHEP1γ, and SHEP1δ (19). The N-terminal SH2 domain is followed by a pro/ser rich domain and a C-terminal GEF-like domain, which has been shown to bind R-Ras, Rap1, and Rap2 (20, 21).
Using conventional and B cell-specific conditional SHEP1-deficient mice, we show that the loss of SHEP1 results in a marked reduction in mature MZ B cells, whereas the MZ B-cell precursor population remains intact. SHEP1-deficient B cells show defects in migration toward CXCL13 and S1P. We found that SHEP1 is required for the basal serine and tyrosine phosphorylation of CasL and for both BCR- and S1P receptor-induced increases in serine/threonine phosphorylation of CasL. We also show that SHEP1 is constitutively bound to CasL in B cells and that this association is required for CasL serine/threonine phosphorylation. These findings establish SHEP1 as an integral component of the signaling networks coordinating MZ B-cell adhesion and migration.
Results
SHEP1 Is Required for Marginal Zone B-Cell Formation.
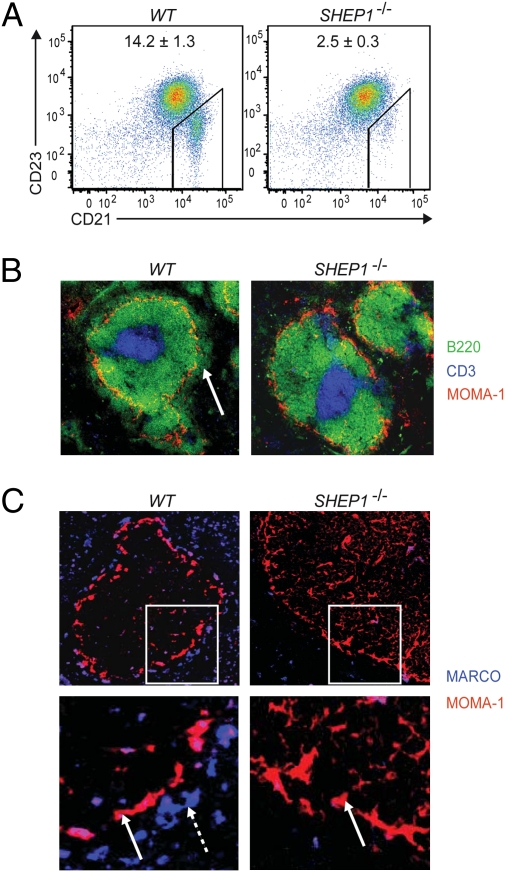
To determine whether SHEP1 is expressed in B cells, purified splenic B cells from wild-type (WT) and newly generated SHEP1−/− mice were lysed and probed by immunoblotting with antibodies that recognize the SH2 domain of SHEP1. The long isoform of SHEP1, SHEP1α, is strongly expressed in B cells, as shown by the 115-kDa band present in WT B-cell lysates, and was not present in SHEP1−/− B-cell lysates (Fig. S1A). To examine B-cell differentiation, flow cytometric analysis of the bone marrow and spleen of SHEP1−/− mice was performed. This analysis revealed no abnormalities in the frequencies of pre-, pro-, immature, and recirculating mature B cells, indicating that early B-cell development is normal in the absence of SHEP1 (Fig. S1B). In the spleen, overall B cell (B220+), T cell (CD3+), and macrophage (CD11b+) frequencies in the SHEP1−/− mice were comparable to WT (Fig. S1C). However, more detailed analysis of SHEP1−/− splenic B-cell subpopulations by flow cytometry and histology revealed a sixfold decrease in the MZ B-cell subset (CD23lo/CD21hi) (Fig. 1 A and B), indicating that SHEP1 is required for MZ B-cell homing, maturation, and/or retention.
Fig. 1.
SHEP1-deficient mice have a disrupted splenic microarchitecture. (A) Splenocytes stained for B220, CD23, and CD21 and analyzed by flow cytometry (B220+ gated cells). The average frequencies of gated MZ B cells (CD23lo, CD21hi) are shown with SEM obtained from six mice. (B) Splenic sections stained for B220 (green), CD3 (blue), and MOMA-1 (red). The marginal zone is indicated by a white arrow. (C) Splenic sections stained for MARCO (blue), which detects MZ macrophages (dashed white arrow), and MOMA-1 (red), which detects metallophilic macrophages (solid white arrow).
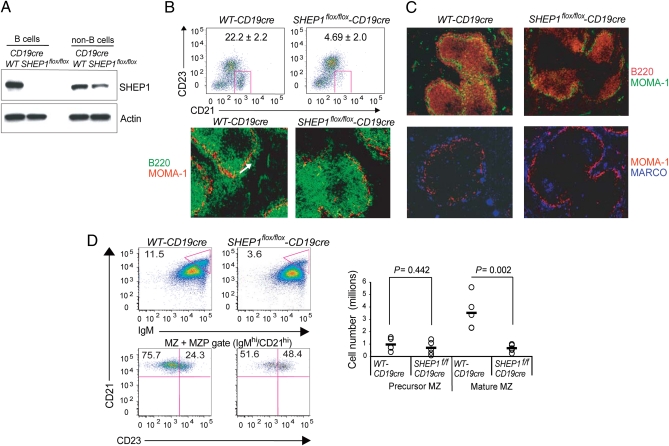
Interestingly, we found that the germline loss of SHEP1 also resulted in a decrease in the MARCO+ macrophages of the MZ but did not affect the MOMA-1+ metallophilic macrophages lining the marginal sinus that circumscribes the follicular zone (Fig. 1C). MZ macrophages and MZ B cells intermingle, and MZ macrophages contribute to the retention and trafficking of B cells in the MZ niche. Thus, to determine whether the observed reduction in MZ B cells was due to a cell autonomous defect, SHEP1flox/flox mice were bred with CD19cre mice to obtain mice bearing a B cell-specific inactivation of the SHEP1 gene. The specific loss of SHEP1 protein in B cells was verified by Western blot (Fig. 2A). Analysis of splenic B-cell subsets by flow cytometry and histology revealed a four- to sixfold decrease in the MZ B-cell population (Fig. 2B) (confirmed by staining for CD1d and CD9 as additional MZ B-cell markers; Fig. S2A). In contrast to our observations in SHEP1−/− mice, the MARCO+ MZ macrophage population in the SHEP1flox/flox-CD19cre mice was unaffected (Fig. 2C). Thus, SHEP1flox/flox-CD19cre mice recapitulate the MZ B-cell phenotype observed in SHEP1−/− mice, indicating that this defect is B cell-intrinsic and that SHEP1 may influence MZ B-cell maturation.
Fig. 2.
B cell-specific SHEP1 deficiency leads to a reduced marginal zone B-cell compartment. (A) Lysates from splenic B cells and non-B cells from WT-CD19cre and SHEP1flox/flox-CD19cre mice immunoblotted for SHEP1 and actin. (B; Upper) WT-CD19cre and SHEP1flox/flox-CD19cre splenic B cells stained for CD23 and CD21. Frequencies of gated MZ B cells are shown with SDs obtained from three mice per group. (Lower) WT-CD19cre and SHEP1flox/flox-CD19cre splenic sections stained for B220 (green) and MOMA-1 (red). MZ is indicated by a white arrow. (C) WT-CD19cre and SHEP1flox/flox-CD19cre splenic sections stained for B220 (red) and MOMA-1 (green; Upper) and MARCO (blue) and MOMA-1 (red; Lower). (D) WT-CD19cre and SHEP1flox/flox-CD19cre splenic cells stained for B220, IgM, CD21, and CD23. The MZ+MZP compartments were gated as CD21hi, IgMhi (Upper Left) and subdivided into CD23hi (precursor MZB) and CD23lo (mature MZB) populations (Lower Left). The dot plot shows total cell numbers from five mice/group (Right). P values were calculated by using Student's t test.
The reduction in MZ B-cell frequency could be due to a reduction in the MZ B-cell precursor (MZP) population or the inability of MZ B cells to situate and remain in the MZ niche. To investigate the first possibility, the frequency of MZ precursor B cells (CD23hi/CD21hi/IgMhi) was assessed. The MZ compartment was subdivided into CD23hi (representing MZ precursor B cells) and CD23lo (representing mature MZ B cells) cells. Although the abundance of mature MZ B cells was reduced, no statistically significant difference was evident in the numbers of MZ precursors in WT-CD19cre versus SHEP1flox/flox-CD19cre mice (Fig. 2D). These findings suggest that SHEP1-deficient B cells traffic normally to the spleen, differentiate to the MZ precursor stage, but fail to complete maturation because of the inability to migrate toward or be retained in the MZ.
SHEP1 Is Required for B-Cell Migration Toward S1P and CXCL13.
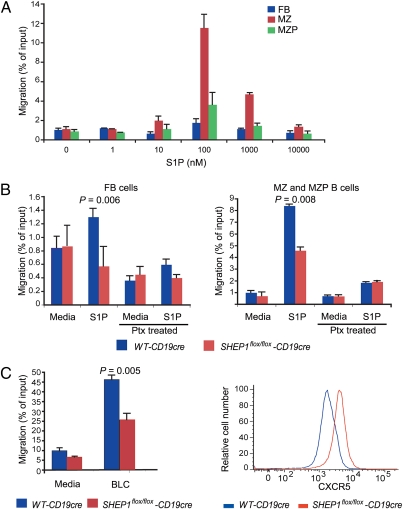
S1P is critical for the chemoattraction and retention of B cells in the MZ niche (4). We found that both mature and MZ precursor B cells migrate toward S1P (Fig. 3A). In the absence of SHEP1, migration of follicular B cells as well as MZ and MZ precursor B cells toward S1P was impaired (Fig. 3B). Treatment with pertussis toxin, a G-protein inhibitor, abolished the migration of both follicular and MZ B cells in response to S1P (Fig. 3B). The reduction in migration toward S1P was likely not due to reduced S1P receptor expression as mRNA transcripts for the S1P receptors were comparable between WT-CD19cre and SHEP1flox/flox-CD19cre B cells (Fig. S2B). Migration toward CXCL13 was also reduced in SHEP1-deficient B cells (Fig. 3C, Left), whereas cell surface expression of CXCR5, the receptor for CXCL13, was not decreased. In fact, CXCR5 expression was higher in SHEP1flox/flox-CD19cre B cells (Fig. 3C, Right). Thus, SHEP1 mediates responses downstream of S1P and CXCR5 receptor engagement to coordinate B-cell chemotaxis.
Fig. 3.
Migration of SHEP1-deficient B cells is impaired in response to BLC/CXCL13 and S1P. (A) Migration of WT B cells in response to different S1P concentrations. The number of follicular (FB), MZ, and MZP B cells in the lower wells was divided by the corresponding number of each B-cell subpopulation in the input wells to obtain a measure of migration efficiency as a percentage of input cells. (B) Migration of WT-CD19cre and SHEP1flox/flox-CD19cre follicular (Left) and MZ+MZP (Right) B cells in response to 100 nM S1P with or without pertussis toxin (Ptx) preincubation. (C) Migration of splenic B cells from WT-CD19cre and SHEP1flox/floxCD19cre mice in response to 1 μg/mL BLC/CXCL13 for 4 h (Left). CXCR5 expression on splenic B cells (Right).
SHEP1 Constitutively Associates with CasL and Promotes Its Hyperphosphorylation.
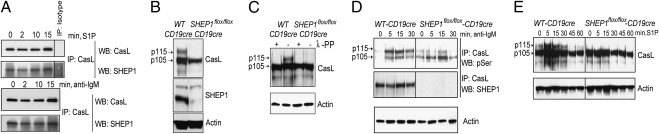
SHEP1 has been shown to associate with CasL, an adaptor protein that promotes integrin activation and is also required for MZ B-cell formation (15). Because integrin function is vital for the retention of MZ B cells (12), we sought to determine whether SHEP1 and CasL functionally interact in B cells. The cellular localization of SHEP1 was determined by immunofluorescence microscopy in unstimulated BAL17 cells, a mature B-cell line. We found that SHEP1 colocalized with the cortical actin ring and appeared to colocalize with CasL (Fig. S3). SHEP1-CasL association was also assessed after stimulation with S1P, or with anti-IgM to stimulate the BCR. CasL was immunoprecipitated from stimulated BAL17 lysates with anti-CasL mAb and immunoblotted for coassociated SHEP1. SHEP1 and CasL exhibited constitutive association, which was maintained over the time course of stimulation with S1P or anti-IgM (Fig. 4A).
Fig. 4.
SHEP1 constitutively associates with CasL, and SHEP1 promotes CasL hyperphosphorylation. (A) Immunoprecipitated CasL from BAL17 cells stimulated with 1 μM S1P (Upper) or 10 μg/mL anti-IgM F(ab’)2 (Lower) and immunoblotted for SHEP1 and CasL. (B) Lysates from splenic B cells from WT-CD19cre and SHEP1flox/flox-CD19cre mice immunoblotted for CasL, SHEP1, and actin. (C) B-cell lysates incubated with or without λ-protein phosphatase (λ-PP) and immunoblotted for CasL and actin. (D) Immunoprecipitated CasL from splenic B cells from WT-CD19cre and SHEP1flox/flox-CD19cre mice stimulated with 10 μg/mL anti-IgM F(ab’)2 and immunoblotted for phosphoserine and SHEP1. Total lysates were immunoblotted for actin. (E) Lysates from splenic B cells from WT-CD19cre and SHEP1flox/flox-CD19cre mice were stimulated with 1 μM S1P and immunoblotted for CasL and actin.
CasL exists as two forms, p105 and p115, as a result of differential phosphorylation. The serine/threonine phosphorylation of p105 at multiple sites converts it to the slower migrating p115 isoform (22), and this hyperphosphorylation is associated with cell adhesion and cytoskeletal organization (23). To investigate the role of SHEP1 in the regulation of CasL phosphorylation, WT-CD19cre and SHEP1flox/flox-CD19cre B-cell lysates were immunoblotted for CasL. SHEP1flox/flox-CD19cre B cells showed a reduction in the 115-kDa band, suggesting a decrease in the hyperphosphorylated form of CasL (Fig. 4B). The reduction in p115 in SHEP1flox/flox-CD19cre B cells cannot be attributed to the exclusive expression of p115 by MZ B cells (which are absent in SHEP1flox/flox-CD19cre mice), because depletion of MZ B cells from WT splenic B-cell preparations did not result in the loss of p115 (Fig. S4A). Furthermore, WT-CD19cre but not SHEP1flox/flox-CD19cre B cells from lymph nodes, which do not have marginal zones, express the p115 form of CasL (Fig. S4B).
Supportive evidence for the phosphorylated status of the p115 form was provided by treatment of lysates from WT-CD19cre or SHEP1flox/flox-CD19cre B cells with λ protein phosphatase followed by immunoblot analysis. This treatment revealed that dephosphorylation of CasL in B cells converts the p115 form into the p105 form (Fig. 4C). To further examine whether CasL phosphorylation is affected by the loss of SHEP1, CasL immunoprecipitates from splenic B cells were immunoblotted with anti-phosphoserine, anti-phosphotyrosine, anti-CasL, and anti-SHEP1 antibodies. We found that SHEP1flox/flox-CD19cre B cells exhibited reduced CasL serine and tyrosine phosphorylation (Fig. S5). Next, we determined whether the p115 form of CasL could be influenced by BCR stimulation. CasL was immunoprecipitated from anti-IgM stimulated splenic B cells and immunoblotted for phosphoserine and SHEP1. We found that the p115 hyperphosphorylated form of CasL was increased upon BCR stimulation, but this modification required the presence of SHEP1 (Fig. 4D). Similarly, S1P receptor stimulation up-regulated the p115 form of CasL in resting WT-CD19cre B cells but not in SHEP1flox/flox-CD19cre B cells (Fig. 4E). These findings indicate that SHEP1 is required for the conversion of p105 CasL into its functionally distinct hyperphosphorylated p115 form.
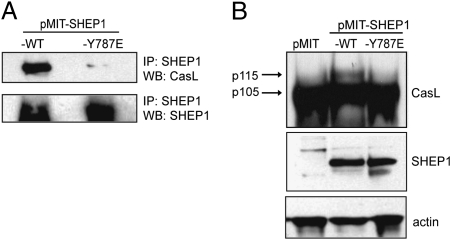
To determine whether SHEP1/CasL association is required for CasL hyperphosphorylation, we generated MSCV-based retroviruses to express either a Y787E mutant (pMIT-SHEP1-Y787E) or WT (pMIT-SHEP1) SHEP1 protein. A mutation in SHEP1β (Y635E) is analogous to Y787E in SHEPα and has been shown to disrupt association with p130Cas (21). SHEP1 was immunoprecipitated from transduced (Thy1.1+) SHEP1flox/flox-CD19cre B cells and immunoblotted for CasL. Although exogenous WT SHEP1 associated with CasL, the SHEP1-Y787E mutant failed to associate, indicating the importance of this residue in the constitutive interaction between SHEP1 and CasL (Fig. 5A). Moreover, although the hyperphosphorylated form of CasL was present in SHEP1flox/flox-CD19cre B cells transduced with pMIT-SHEP1, this form was absent in SHEP1flox/flox-CD19cre B cells infected with pMIT-SHEP1-Y787E or pMIT alone (Fig. 5B). Thus, it is likely that direct binding of SHEP1 to CasL is necessary for CasL hyperphosphorylation.
Fig. 5.
Direct interaction between SHEP1 and CasL is required for hyperphosphorylation of CasL. (A) SHEP1flox/flox-CD19cre B cells were transduced with pMIT-SHEP1-WT or with pMIT-SHEP1-Y787E. Immunoprecipitated SHEP1 from sorted Thy1.1+ cells immunoblotted for CasL and SHEP1. (B) Lysates from Thy1.1+ SHEP1flox/flox-CD19cre B cells were transduced with pMIT, pMIT-SHEP1-WT, or pMIT-SHEP1-Y787E and immunoblotted for CasL, SHEP1, and actin.
Discussion
Marginal zone B cells occupy a strategic niche in the spleen, where they are exposed to antigens delivered via the blood sinuses. Engineered mice bearing mutations that impair BCR signaling often present defects in MZ B-cell maturation, implicating BCR signal strength in the generation and maintenance of this B-cell subset (2). Notably, we did not observe an impairment in Akt and Erk phosphorylation or in calcium flux after BCR stimulation of SHEP1-deficient cells (Fig. S6), indicating a selective downstream defect in BCR signaling. Upon antigen encounter, MZ B cells rapidly differentiate into antibody-producing cells (24). MZ B cells can also deliver opsonized antigen to follicular dendritic cells in an antigen-nonspecific manner via the binding of complement receptors (25–27). The continuous movement of B cells from the MZ to the follicle is thought to depend on differential responsiveness and desensitization to CXCL13 versus S1P (26). Indeed, MZ B-cell formation is normal in S1P1−/− mice, but the cells are displaced to the follicles (4). Deficiencies in downstream signaling components that promote migration and adhesion, such as DOCK2, Lsc, or CasL, lead to the loss of MZ B cells (10, 11, 15). These deficiencies cause developmental defects in MZ B-cell formation rather than failed localization. Overall, these findings underscore a strong dependence of MZ B cells on integrin activation and actin reorganization. Our observation that MZ B cells are absent in SHEP1-deficient mice prompted us to investigate the underlying mechanisms of this B cell-intrinsic defect. Because SHEP1 has been shown to regulate the migration of COS cells and T cells (21, 28), we reasoned that SHEP1 might also regulate B-cell migration. Indeed, in the absence of SHEP1, B cells revealed impaired migration toward CXCL13 and S1P.
Mechanistically, we show that SHEP1 mediates S1P and B-cell receptor signals by associating with and promoting the hyperphosphorylation of CasL, a key scaffolding molecule that mediates integrin signaling, cell adhesion, and cell migration (29). CasL, and the family member p130Cas, contain multiple domains that serve as docking sites for molecules involved in integrin signaling and actin reorganization. The N-terminal SH3 domain of CasL binds the proline-rich region of FAK and Pyk2 (30, 31). The tyrosine-phosphorylated sites in the substrate domain of CasL recruit CrkL, another key adaptor molecule that, upon tyrosine phosphorylation, engages the exchange factors C3G and DOCK2 (17, 32). Our finding that CasL tyrosine phosphorylation is reduced in the absence of SHEP1 suggests that CrkL recruitment may be impaired. It has been shown that the C-terminal GEF-like domain of SHEP1 binds certain Ras family GTPases (20) and p130Cas (21, 33). We show here that SHEP1 binds CasL, and that this interaction depends on Y787 located in the GEF-like domain of SHEP1. This C-terminal-to-C-terminal association of SHEP1 and CasL could potentially expose docking sites for kinases and phosphatases that regulate adhesion and migration.
The function of the serine-rich domain of p130Cas or CasL has been linked to the 14-3-3 proteins, which only bind to Cas family proteins when their serine phosphorylation is induced by sustained integrin engagement with extracellular matrix ligands (34, 35). Furthermore, structural analysis of p130Cas revealed that the serine-rich domain is a four-helical-bundle repeat that bears structural similarity to the FAT domains of FAK and Pyk2 (36), both of which bind paxillin at focal adhesion sites (37). Recently, it has been shown that paxillin and p130Cas form a complex with Crk, which activates Rac1 and facilitates pressure-induced adhesion (38). These findings suggest that the serine-rich domain of CasL may provide an important platform for protein interactions that regulate adhesion and motility. Indeed, the p115 isoform of CasL, representing its hyperphosphorylated form, has been shown to be induced by substrate adhesion in fibroblasts and to become dephosphorylated upon detachment, indicating that the interconversion of p105 to p115 is dynamically regulated by cell adhesion and detachment (23, 39).
We found that CasL and SHEP1 constitutively associate in B cells and that this direct association is required for the generation of the hyperphosphorylated p115 CasL. p115 CasL is likely necessary for the integration of BCR and S1P-dependent integrin signaling that permits MZ B cells to be retained in the MZ, whereas dephosphorylation of CasL contributes to cell detachment and trafficking to the follicle. Thus, the current studies of SHEP1 function lend molecular insight into the dynamic nature of MZ B-cell migration and adhesion.
Methods
Mice.
The SHEP1−/− and SHEP1flox/flox mice have been recently described (40). SHEP1flox/flox mice were bred with CD19cre mice (41) to inactivate SHEP1 in the B lineage. Mice were genotyped by PCR amplification of genomic DNA from tail samples using the listed primers (Table S1). Animals were maintained in a pathogen-free animal facility at the Sanford-Burnham Medical Research Institute (SBMRI), which approved all animal procedures through the Institutional Animal Care and Use Committee.
RT-PCR, Cloning, and Mutagenesis.
Splenic and lymph node B cells were purified by using anti-CD43–conjugated magnetic beads (Miltenyi Biotec). Total RNA was isolated from B cells by using Nucleospin RNAII kit (Macherey-Nagel). For cloning and mutagenesis of SHEP1, total B-cell cDNA was prepared by using the MMLV Reverse Transcriptase cDNA Advantage Kit (Clontech), mutagenized by using the QuikChange Lightning Site-Directed Mutagenesis Kit (Stratagene), and cloned into the bicistronic, retroviral vector pMIT (MSCV-IRES-Thy1.1). Primers used for RT-PCR and mutagenesis are listed in Table S1.
Cells and Retroviral Transduction.
BAL17 cells were grown in 10% FBS/RPMI 1640 complete medium. Retroviral supernatants were obtained by transfecting the packaging cell line, Phoenix-eco, with Lipofectamine 2000 (Invitrogen). For spinfections, primary B cells were cultured for 18–24 h with mitogens before infection. Viral supernatants were incubated with 8 μg/mL polybrene (Sigma) for 10 min on ice before addition to cells, and cells were spinfected at 930 g for 1.5 h at 30 °C. After spinfection, cells were resuspended in fresh media containing mitogens. Two days after infection, B cells were harvested and analyzed.
Immunoblotting.
One to 10 million cells were stimulated with 10 μg/mL goat anti-mouse IgM F(ab′)2 (Jackson ImmunoResearch Laboratories) or indicated concentrations of S1P (Biomol International) for the indicated times at 37 °C. Before stimulation, primary B cells were rested for 30–60 min in Hanks’ Balanced Salt Solution (HBSS; GIBCO). Cell pellets were lysed on ice for 30 min in either RIPA or Nonidet P-40 lysis buffer plus protease and phosphatase inhibitors (2 μg/mL leupeptin, 2 mM PMSF, 2 μg/mL aprotinin, 1 mM sodium orthovanadate, and 50 mM NaF). Lysates were resolved by using precast 4–12% polyacrylamide Bis-Tris or 3–8% Tris-Acetate gels (Bio-Rad) and transferred onto nitrocellulose membranes. For λ protein phosphatase treatment, lysates were prepared without phosphatase inhibitors and EDTA, and the dephosphorylation reaction was performed for 1 h at 30 °C as indicated by the manufacturer (Sigma). Antibody against CasL was from Rockland Immunochemicals, and antibodies against Akt, phospho-Akt, Erk, phospho-Erk, and β-actin were from Cell Signaling Technology. Generation of the SHEP1 polyclonal antiserum has been described (40). Protein bands were revealed with HRP-labeled donkey anti-mouse or anti-rabbit antibodies (Jackson ImmunoResearch Laboratories) and developed with a chemiluminescence kit (SuperSignal West Pico; Thermo Scientific).
Flow Cytometry.
Single-cell spleen suspensions were stained with the following conjugated antibodies/reagents from eBiosciences: IgM-APC, B220-PerCP-Cy5.5, CD11b-PE-Cy7, CD23-FITC, CD21-PE, CD9-biotin, CD1d-biotin, and streptavidin-PE-Cy7, and analyzed by using a FACSCanto flow cytometer (BD Biosciences) and FlowJo software (Treestar). For intracellular staining, B cells were prestained for CD23, stimulated, fixed with BD Biosciences Cytofix fixation buffer, permeabilized with BD Phosphoflow Perm/Wash buffer, and stained with anti-phospho Akt followed by surface staining with anti-B220 and anti-CD21.
Histology.
Eight-micrometer frozen spleen sections were acetone-fixed and stained with reagents from eBiosciences (B220-FITC, IgM-APC, CD3-APC, and streptavidin-Cy5) or from BMA Biomedicals (MOMA-1-biotin). Sections were mounted with Gel/Mount (Biomeda) and sealed with glass coverslips. Images were acquired by using Zeiss Axiocam M1 microscope (Zeiss) and Slidebook software (Intelligent Imaging Innovations). For SHEP1 and CasL intracellular staining, cells were fixed in 2% formaldehyde, permeabilized with 0.5% Triton X-100, and stained with SHEP1 and CasL antibodies followed by Cy5- or PE-conjugated secondary antibodies, phalloidin-FITC, and DAPI.
Transwell Migration Assay.
Two million splenic B cells were placed in insert wells of a Transwell plate (Corning; 5.0 μm in diameter, 24-well plate) in RPMI 1640 containing 1% FBS and placed in wells containing 1 μg/mL CXCL13. Cells were allowed to migrate for 4 h at 37 °C. For migration toward S1P, splenic B cells were preincubated at 37 °C in RPMI 1640 containing 1% BSA for 30 min with or without 100 ng/mL pertussis toxin (Calbiochem). Two million preincubated cells were placed in insert wells. Insert wells were placed in wells containing the indicated concentrations of S1P. Migrant cells as well as input cells were stained with antibodies specific for B220, CD23, CD21, and IgM. The numbers of migrant cells were divided by input cell numbers to obtain migration efficiency.
Note Added in Proof.
While this paper was in review, Al-Shami et al. (42) published an independent line of SHEP1(Sh2d3c)−/− mice that were found to lack marginal zone B cells.
Supplementary Material
Acknowledgments
We thank V. Vervoort (SBMRI), S. Roselli (SBMRI), and L. Wang (SBMRI) for characterizing the SHEP1 knockout mice and providing animals for some of the experiments, and L. Anderson (BD Biosciences, La Jolla, CA) for assistance with intracellular staining for phospho-Akt. This work was supported by National Institutes of Health Grants AI059447 (to R.C.R.) and CA102583 (to E.B.P.). C.D.B. was supported by National Institutes of Health Supplemental Grant AI059447 and by the Howard Hughes Medical Institute Med-Into-Grad program; M.M.H. was supported by the Fishman Fund and the Florence Barach Postdoctoral Fellowship; and Y.W. was supported by a postdoctoral fellowship from the Fondation pour la Recherche Médicale.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1007558107/-/DCSupplemental.
References
- 1.Allen CD, Cyster JG. Follicular dendritic cell networks of primary follicles and germinal centers: Phenotype and function. Semin Immunol. 2008;20:14–25. doi: 10.1016/j.smim.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pillai S, Cariappa A. The follicular versus marginal zone B lymphocyte cell fate decision. Nat Rev Immunol. 2009;9:767–777. doi: 10.1038/nri2656. [DOI] [PubMed] [Google Scholar]
- 3.Rieken S, et al. Lysophospholipids control integrin-dependent adhesion in splenic B cells through G(i) and G(12)/G(13) family G-proteins but not through G(q)/G(11) J Biol Chem. 2006;281:36985–36992. doi: 10.1074/jbc.M605287200. [DOI] [PubMed] [Google Scholar]
- 4.Cinamon G, et al. Sphingosine 1-phosphate receptor 1 promotes B cell localization in the splenic marginal zone. Nat Immunol. 2004;5:713–720. doi: 10.1038/ni1083. [DOI] [PubMed] [Google Scholar]
- 5.An S, Goetzl EJ, Lee H. Signaling mechanisms and molecular characteristics of G protein-coupled receptors for lysophosphatidic acid and sphingosine 1-phosphate. J Cell Biochem Suppl. 1998;30-31:147–157. [PubMed] [Google Scholar]
- 6.Wang F, Nobes CD, Hall A, Spiegel S. Sphingosine 1-phosphate stimulates rho-mediated tyrosine phosphorylation of focal adhesion kinase and paxillin in Swiss 3T3 fibroblasts. Biochem J. 1997;324:481–488. doi: 10.1042/bj3240481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Y, et al. A critical role of Rap1b in B-cell trafficking and marginal zone B-cell development. Blood. 2008;111:4627–4636. doi: 10.1182/blood-2007-12-128140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duchniewicz M, et al. Rap1A-deficient T and B cells show impaired integrin-mediated cell adhesion. Mol Cell Biol. 2006;26:643–653. doi: 10.1128/MCB.26.2.643-653.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsukamoto N, Hattori M, Yang H, Bos JL, Minato N. Rap1 GTPase-activating protein SPA-1 negatively regulates cell adhesion. J Biol Chem. 1999;274:18463–18469. doi: 10.1074/jbc.274.26.18463. [DOI] [PubMed] [Google Scholar]
- 10.Fukui Y, et al. Haematopoietic cell-specific CDM family protein DOCK2 is essential for lymphocyte migration. Nature. 2001;412:826–831. doi: 10.1038/35090591. [DOI] [PubMed] [Google Scholar]
- 11.Rubtsov A, et al. Lsc regulates marginal-zone B cell migration and adhesion and is required for the IgM T-dependent antibody response. Immunity. 2005;23:527–538. doi: 10.1016/j.immuni.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 12.Lu TT, Cyster JG. Integrin-mediated long-term B cell retention in the splenic marginal zone. Science. 2002;297:409–412. doi: 10.1126/science.1071632. [DOI] [PubMed] [Google Scholar]
- 13.Lo CG, Lu TT, Cyster JG. Integrin-dependence of lymphocyte entry into the splenic white pulp. J Exp Med. 2003;197:353–361. doi: 10.1084/jem.20021569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Astier A, et al. Association of the Cas-like molecule HEF1 with CrkL following integrin and antigen receptor signaling in human B-cells: Potential relevance to neoplastic lymphohematopoietic cells. Leuk Lymphoma. 1997;28:65–72. doi: 10.3109/10428199709058332. [DOI] [PubMed] [Google Scholar]
- 15.Seo S, et al. Crk-associated substrate lymphocyte type is required for lymphocyte trafficking and marginal zone B cell maintenance. J Immunol. 2005;175:3492–3501. doi: 10.4049/jimmunol.175.6.3492. [DOI] [PubMed] [Google Scholar]
- 16.Nolz JC, et al. The WAVE2 complex regulates T cell receptor signaling to integrins via Abl- and CrkL-C3G-mediated activation of Rap1. J Cell Biol. 2008;182:1231–1244. doi: 10.1083/jcb.200801121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nishihara H, et al. DOCK2 associates with CrkL and regulates Rac1 in human leukemia cell lines. Blood. 2002;100:3968–3974. doi: 10.1182/blood-2001-11-0032. [DOI] [PubMed] [Google Scholar]
- 18.Minegishi M, et al. Structure and function of Cas-L, a 105-kD Crk-associated substrate-related protein that is involved in beta 1 integrin-mediated signaling in lymphocytes. J Exp Med. 1996;184:1365–1375. doi: 10.1084/jem.184.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vervoort VS, Roselli S, Oshima RG, Pasquale EB. Splice variants and expression patterns of SHEP1, BCAR3 and NSP1, a gene family involved in integrin and receptor tyrosine kinase signaling. Gene. 2007;391:161–170. doi: 10.1016/j.gene.2006.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dodelet VC, Pazzagli C, Zisch AH, Hauser CA, Pasquale EB. A novel signaling intermediate, SHEP1, directly couples Eph receptors to R-Ras and Rap1A. J Biol Chem. 1999;274:31941–31946. doi: 10.1074/jbc.274.45.31941. [DOI] [PubMed] [Google Scholar]
- 21.Dail M, et al. SHEP1 function in cell migration is impaired by a single amino acid mutation that disrupts association with the scaffolding protein cas but not with Ras GTPases. J Biol Chem. 2004;279:41892–41902. doi: 10.1074/jbc.M402929200. [DOI] [PubMed] [Google Scholar]
- 22.Law SF, Zhang YZ, Klein-Szanto AJ, Golemis EA. Cell cycle-regulated processing of HEF1 to multiple protein forms differentially targeted to multiple subcellular compartments. Mol Cell Biol. 1998;18:3540–3551. doi: 10.1128/mcb.18.6.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng M, McKeown-Longo PJ. Cell adhesion regulates Ser/Thr phosphorylation and proteasomal degradation of HEF1. J Cell Sci. 2006;119:96–103. doi: 10.1242/jcs.02712. [DOI] [PubMed] [Google Scholar]
- 24.Oliver AM, Martin F, Kearney JF. IgMhighCD21high lymphocytes enriched in the splenic marginal zone generate effector cells more rapidly than the bulk of follicular B cells. J Immunol. 1999;162:7198–7207. [PubMed] [Google Scholar]
- 25.Ferguson AR, Youd ME, Corley RB. Marginal zone B cells transport and deposit IgM-containing immune complexes onto follicular dendritic cells. Int Immunol. 2004;16:1411–1422. doi: 10.1093/intimm/dxh142. [DOI] [PubMed] [Google Scholar]
- 26.Cinamon G, Zachariah MA, Lam OM, Foss FW, Jr, Cyster JG. Follicular shuttling of marginal zone B cells facilitates antigen transport. Nat Immunol. 2008;9:54–62. doi: 10.1038/ni1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gray D, Kumararatne DS, Lortan J, Khan M, MacLennan IC. Relation of intra-splenic migration of marginal zone B cells to antigen localization on follicular dendritic cells. Immunology. 1984;52:659–669. [PMC free article] [PubMed] [Google Scholar]
- 28.Regelmann AG, Danzl NM, Wanjalla C, Alexandropoulos K. The hematopoietic isoform of Cas-Hef1-associated signal transducer regulates chemokine-induced inside-out signaling and T cell trafficking. Immunity. 2006;25:907–918. doi: 10.1016/j.immuni.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 29.Seo S, Ichikawa M, Kurokawa M. Structure and function of cas-L and integrin-mediated signaling. Crit Rev Immunol. 2006;26:391–406. doi: 10.1615/critrevimmunol.v26.i5.20. [DOI] [PubMed] [Google Scholar]
- 30.van Seventer GA, et al. Focal adhesion kinase regulates beta1 integrin-dependent T cell migration through an HEF1 effector pathway. Eur J Immunol. 2001;31:1417–1427. doi: 10.1002/1521-4141(200105)31:5<1417::AID-IMMU1417>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 31.Lakkakorpi PT, et al. Stable association of PYK2 and p130(Cas) in osteoclasts and their co-localization in the sealing zone. J Biol Chem. 1999;274:4900–4907. doi: 10.1074/jbc.274.8.4900. [DOI] [PubMed] [Google Scholar]
- 32.Smit L, van der Horst G, Borst J. Sos, Vav, and C3G participate in B cell receptor-induced signaling pathways and differentially associate with Shc-Grb2, Crk, and Crk-L adaptors. J Biol Chem. 1996;271:8564–8569. doi: 10.1074/jbc.271.15.8564. [DOI] [PubMed] [Google Scholar]
- 33.Derunes C, et al. Molecular determinants for interaction of SHEP1 with Cas localize to a highly solvent-protected region in the complex. FEBS Lett. 2006;580:175–178. doi: 10.1016/j.febslet.2005.11.070. [DOI] [PubMed] [Google Scholar]
- 34.Takala H, et al. Beta2 integrin phosphorylation on Thr758 acts as a molecular switch to regulate 14-3-3 and filamin binding. Blood. 2008;112:1853–1862. doi: 10.1182/blood-2007-12-127795. [DOI] [PubMed] [Google Scholar]
- 35.Garcia-Guzman M, Dolfi F, Russello M, Vuori K. Cell adhesion regulates the interaction between the docking protein p130(Cas) and the 14-3-3 proteins. J Biol Chem. 1999;274:5762–5768. doi: 10.1074/jbc.274.9.5762. [DOI] [PubMed] [Google Scholar]
- 36.Briknarová K, et al. The serine-rich domain from Crk-associated substrate (p130cas) is a four-helix bundle. J Biol Chem. 2005;280:21908–21914. doi: 10.1074/jbc.M501258200. [DOI] [PubMed] [Google Scholar]
- 37.Collins M, Bartelt RR, Houtman JC. T cell receptor activation leads to two distinct phases of Pyk2 activation and actin cytoskeletal rearrangement in human T cells. Mol Immunol. 2010;47:1665–1674. doi: 10.1016/j.molimm.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 38.Downey C, Craig DH, Basson MD. Pressure activates colon cancer cell adhesion via paxillin phosphorylation, Crk, Cas, and Rac1. Cell Mol Life Sci. 2008;65:1446–1457. doi: 10.1007/s00018-008-8038-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zheng M, McKeown-Longo PJ. Regulation of HEF1 expression and phosphorylation by TGF-beta 1 and cell adhesion. J Biol Chem. 2002;277:39599–39608. doi: 10.1074/jbc.M202263200. [DOI] [PubMed] [Google Scholar]
- 40.Roselli S, Wallez Y, Wang L, Vervoort V, Pasquale EB. The SH2 domain protein Shep1 regulates the in vivo signaling function of the scaffolding protein Cas. Cell Signal. 2010;22:1745–1752. doi: 10.1016/j.cellsig.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rickert RC, Roes J, Rajewsky K. B lymphocyte-specific, Cre-mediated mutagenesis in mice. Nucleic Acids Res. 1997;25:1317–1318. doi: 10.1093/nar/25.6.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Al-Shami A, et al. The adaptor protein Sh2d3c is critical for marginal zone B cell development and function. J Immunol. 2010;185:327–334. doi: 10.4049/jimmunol.1000096. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.