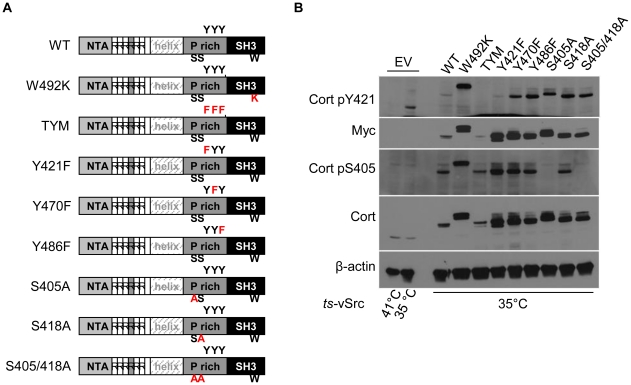
Figure 4. Cortactin tyrosine and serine phosphorylation resultant of v-Src activation are not interdependent.
(A) Schematic diagram of the cortactin point mutant constructs assayed for phosphorylation. Mutated codons are denoted on the left and displayed with the corresponding mutant amino acid at the appropriate position within cortactin in red. (B) Murine fibroblasts lacking endogenous Src, Yes and Fyn (SYF) were transfected with murine-specific cortactin siRNA and cultured for 48 h to deplete endogenous cortactin. Cells were subsequently co-transected with the temperature-sensitive v-Src construct La29 (tsLa29) and wild-type or the indicated myc-tagged human cortactin point-mutant constructs at 41°C (non-permissive temperature). TPM; triple point mutant consisting of Y-F mutations at positions 421, 470 and 486. After transfection, cells were cultured at 41°C, then shifted to 35°C (permissive temperature) for 2 h to promote v-Src activation. Recombinant cortactin proteins were assayed by immunoblotting with anti-cortactin-pY421, anti-cortactin-pS405, anti-myc, anti-cortactin, and anti-beta-actin antibodies. Note that the inability of cortactin to be phosphorylated on Y421 does not impact its ability to be phosphorylated on S405, nor does lack of S405 phosphorylation impact Y421 phosphorylation.