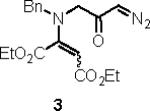
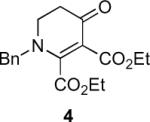
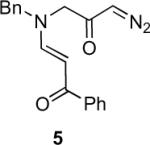
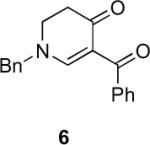
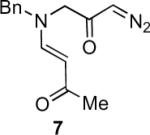
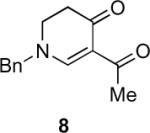
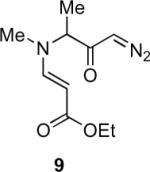
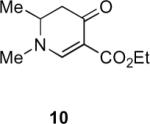
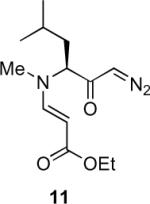
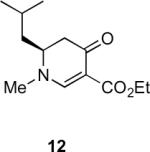
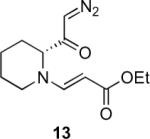
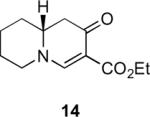
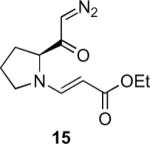
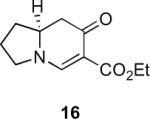
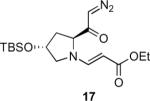
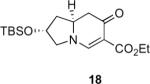
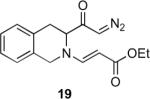
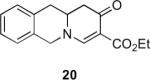
Table 1.
Substrate scope of the reactiona
Reaction conditions: diazoketone and PhCO2Ag (10 mol%) in CH2Cl2 (0.2 M).
20 mol% of PhCO2Ag was used.
ee was determined by 19F NMR (Mosher ester derivatives); only one isomer was observed.
Ag2O (10 mol%) and C2H4Cl2 (0.2 M) were used.
dr was determined by 1H NMR; only one isomer was observed.