Abstract
In animals, repeated administration of 3,4-methylenedioxymethamphetamine (MDMA) reduces markers of serotonergic activity and studies show similar serotonergic deficits in human MDMA users. Using proton magnetic resonance spectroscopy (1H-MRS) at 11.7 Tesla, we measured the metabolic neurochemical profile in intact, discrete tissue punches taken from prefrontal cortex, anterior striatum, and hippocampus of rats administered MDMA (5 mg/kg IP, 4× q 2 h) or saline and euthanized 7 days after the last injection. Monoamine content was measured with HPLC in contralateral punches from striatum and hippocampus to compare the MDMA-induced loss of 5HT innervation with constituents in the 1H-MRS profile. When assessed 7 days after the last MDMA injection, levels of hippocampal and striatal serotonin (5HT) were significantly reduced, consistent with published animal studies. N-acetylaspartate (NAA) levels were significantly increased in prefrontal cortex and not affected in anterior striatum or hippocampus; myo-inositol (INS) levels were increased in prefrontal cortex and hippocampus but not anterior striatum. Glutamate levels were increased in prefrontal cortex and decreased in hippocampus, while GABA levels were decreased only in hippocampus. The data suggest that NAA may not reliably reflect MDMA-induced 5HT neurotoxicity. However, the collective pattern of changes in 5HT, INS, glutamate and GABA is consistent with persistent hippocampal neuroadaptations caused by MDMA.
Keywords: Magnetic Resonance Spectroscopy, MDMA, Neurotoxicity, Glutamate, Hippocampus, Rat
1. Introduction
Several studies, using both humans and animals, have sought to determine if 3,4-methylenedioxymethamphetamine (MDMA) produces permanent changes in brain structure and function. Animal studies show that MDMA reduces brain levels of serotonin (5HT), tryptophan hydroxylase, and the serotonin reuptake transporter (SERT or 5HTT) (Battaglia et al., 1987, Commins et al., 1987, O'Hearn et al., 1988, Ricaurte et al., 1988, Slikker et al., 1988), as well as increasing mitochondrial oxidative stress and microglial activation (Kuhn and Geddes, 2000, Thomas et al., 2004, Yamamoto and Raudensky, 2008). These findings support the hypothesis that MDMA is neurotoxic to serotonin neurons; however, the effect of MDMA on 5HT neurons is observed in the absence of glial activation (Fantegrossi et al., 2008, Wang et al., 2004). Human neuroimaging studies have focused on measurements of 5HT markers, neuronal function, and viability (reviewed by (Cowan, 2007, Cowan et al., 2008)). For example, positron emission tomography studies have uncovered similar findings as that seen in animals, showing lower levels of SERT and altered 5HT receptor expression in active or abstinent human MDMA users (McCann et al., 1998, McCann et al., 2005, Reneman et al., 2002a, Reneman et al., 2002b, Ricaurte et al., 1988, Scheffel et al., 1998). On the other hand, results from magnetic resonance spectroscopy studies on MDMA users or abstinent users are inconsistent in regards to levels of N-acetylaspartate (NAA) and myo-inositol (INS), which are indirect markers of neuronal and glial cell integrity, respectively (Chang et al., 1999, Cowan et al., 2007, Daumann et al., 2004, Obergriesser et al., 2001, Reneman et al., 2002c, Reneman et al., 2001). The fact that 5HT markers are consistently reduced by MDMA in animals and humans while glial activity markers are not consistently changed by MDMA requires further study to determine the nature of MDMA induced neurotoxicity. Furthermore, it is important to determine if proton-magnetic resonance spectroscopy (1H-MRS) can detect MDMA-induced loss of 5HT containing neurons or axonal projections.
Proton-MRS provides a chemical shift spectrum of MR-visible chemicals in a biological matrix by first suppressing the prominent signal from water protons and then measuring signals from chemicals that have MR-visible protons. In the brain, MR-visible compounds include γ-amino-butyric acid (GABA), glutamate (GLU), and glutamine (GLN) in addition to INS and NAA (Moore and Galloway, 2002, Shulman et al., 1993); however most clinical 1H-MRS studies focus on INS and NAA because of sensitivity limitations associated with clinical magnets. In humans, this non-invasive technique has revealed decreased levels of the neuronal marker NAA in human depression (Sharma et al., 1992), epilepsy (Duc et al., 1998, Hajek et al., 1998, Mendes-Ribeiro et al., 1998), schizophrenia (Maier et al., 1995, Sharma et al., 1992) and Parkinson’s disease (Camicioli et al., 2007). Despite the neurotoxic effect of MDMA on 5HT neurons and the potential to discern this effect with 1H-MRS (i.e. alterations in NAA levels), studies in past MDMA users show inconsistent results regarding NAA levels, with a decrease or no change in the neuronal marker (reviewed by (Cowan, 2007, Cowan et al., 2008)). Potential changes in NAA under controlled conditions of MDMA-induced 5HT deficits remain to be determined. In order to consider NAA as a marker sensitive to MDMA induced neurotoxicity, controlled preclinical studies must first establish the relationship between these 2 agents.
The inconsistencies of human studies investigating MDMA related neurotoxicity and the indications in animal studies that deficits in 5HT markers may not reflect neuronal loss or axonal degeneration, suggest that MDMA neurotoxicity is complex. Structural alterations in medium spiny neurons of the striatum and neurons in the prefrontal cortex (Ball et al., 2009, Schmued, 2003), which are unlikely to be serotonin-containing neurons, may be related to the observed decrease in NAA in a few human 1H-MRS studies. Confounding factors like polydrug use or pre-existing stress may contribute directly to neurotoxicity or be additive to the effects of MDMA observed in humans.
In this study rats were treated with MDMA using an established neurotoxic regimen (i.e. serotonin-depleting) (Fantegrossi et al., 2008) to determine if MDMA administered under a controlled setting in laboratory rats affects NAA levels. The primary hypothesis was that if 1H-MRS can detect MDMA-induced loss of 5HT containing neurons or axonal projections, then the neuronal marker NAA will be decreased in these areas. Additionally, this study was done to determine if a serotonin-depleting regimen of MDMA causes long-lasting changes in brain GLU and GABA levels using 1H-MRS since monoamines modulate the synaptic efficacy of the amino acid neurotransmitters (Sesack and Grace, 2010). Finally, additional neurometabolome information measured by 1H-MRS was used to make inferences about functional tone of neurotransmitters.
2. Materials and Methods
2.1 Animal Welfare, Research Materials, and Experimental Paradigm
Male Sprague-Dawley rats were group housed four per cage in standard microisolator rat cages. Housing conditions, including light cycle (12 h light/dark cycle; on 7 AM off 7 PM), temperature (~24°C) and humidity (35–40%), were controlled and food and water was available ad libitum. Rats were acclimated to the new environment for at least 5 days before testing. Rats were handled and weighed daily throughout the experiment. Eighteen rats were split into two experimental groups (saline and MDMA); samples sizes are stated in the figure legends. All experimental procedures were approved by the Wayne State University Institutional Animal Care and Use Committee. The Division of Laboratory Animal Resources maintains AAALAC accredited animal facilities, and animals are cared for in accordance with applicable portions of the Animal Welfare Act and “Guide for the Care and Use of Laboratory Animals”.
All supplies where purchased from Bruker Biospin Corp. (Billerica, MA), ESA Biosciences (Chelmsford, MA), Sigma-Aldrich (St. Louis, MO), or Fisher Scientific (Pittsburgh, PA). Rats were purchased from Charles River Laboratories (Wilmington, MA).
Racemic (±)MDMA (5 mg/kg; dissolved in saline) or saline (1 ml/kg; 0.9% NaCl) was injected intraperitoneally (IP; 1× body weight) every 2 hours for a total of 4 injections using an established dosing paradigm (Fantegrossi et al., 2008, Perrine et al., 2008). Animals were euthanized by rapid decapitation without anesthesia 7 days after the last injection to obtain brain tissue samples. Brains were rapidly removed, placed into an ice-cold brain matrix, and sliced into 2 mm coronal slices. Coronal slices were placed on a block of solid CO2, and using a 1.5 mm diameter tissue biopsy-punch, tissue punches were taken from individual slices containing regions of interest. Regions of interest included medial prefrontal cortex, anterior dorsal striatum, and dorsal hippocampus (Paxinos and Watson, 2006). A single medial punch was taken for the prefrontal cortex and used for 1H-MRS analysis; two bilateral tissue punches were taken from anterior striatum and hippocampus and one used for 1H-MRS and the other used for high pressure liquid chromatography (HPLC) analysis. Tissue samples were stored at −80°C until use. The drug regimen did not cause a significant decrease in weight gain between groups when determined 7 days after drug treatment (saline = 386±5 g, MDMA = 368±9 g; P>0.05). Core body temperature was not recorded during drug treatment.
2.2 High Pressure Liquid Chromatography
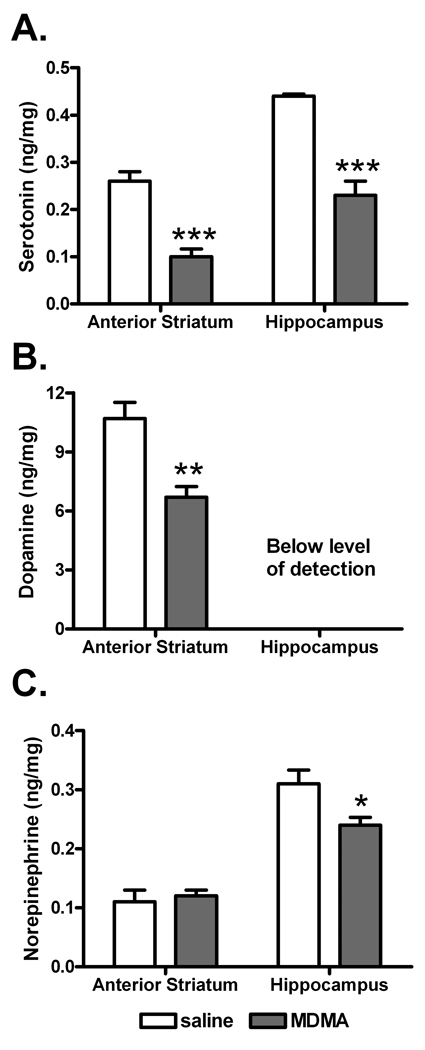
Monoamine tissue levels were determined using previously described HPLC techniques (Koch and Galloway, 1997, Perrine et al., 2008). The tissue punches were weighed, sonically disrupted in 200 µL of 200 mM HClO4, and centrifuged for 5 minutes to remove cellular debris. An aliquot of the supernatant was placed in an ESA 542 autoinjector (ESA Biosciences, Chelmsford, MA) maintained at 4°C and a portion injected onto a C18-RP column (30°C) with ESA mobile phase (MD-TM; 75 mM sodium dihydrogen phosphate monohydrate, 1.7 mM 1-octanesulfonic acid sodium salt, 100 µl/l triethylamine, 25 µM ethylenediaminetetraacetic acid, 10% acetonitrile and pH=3 with phosphoric acid) running at a flow rate of 0.6 ml/min. Coulometric detection was performed with an ESA 5011A dual electrode cell (220 mV) and signals analyzed on an EZChrome Elite data processing platform. Absolute tissue values for serotonin, dopamine and norepinephrine were determined by comparison with external standard curves and corrected for tissue weight. Data are presented as mean ± standard error of the mean (s.e.m.) and are expressed as ng monoamine / mg tissue (figure 2).
Figure 2. Effects of MDMA on monoamine neurotransmitter levels.
The effects of MDMA administration on rat striatal and hippocampal levels of serotonin (A), dopamine (B) and norepinephrine (C) were determined seven days after repeated injection (IP every 2 hours, 4 injections). Sample sizes (N) were 9 rats per group, except for anterior striatum dopamine where saline=7 and MDMA=8. Data are shown as mean ± standard error of the mean. Data were analyzed by independent t-test (*P<0.05, **P<0.01, and ***P<0.001 for saline versus MDMA).
2.3 1H-MRS
A specialized application of 1H-MRS, High Resolution-Magic Angle Spinning (HR-MAS) 1H-MRS was applied to intact brain samples to provide highly resolved resonance peaks at 11.7 Tesla (T). HR-MAS 1H-MRS minimizes dipole-dipole interactions by rapidly spinning the sample around its own axis while positioned at 54.7° (magic angle) relative to the static magnetic field (Bo). This specialized application greatly improves sensitivity and provides high resolution of the chemical shift spectrum when compared to clinical 1H-MRS. Under the conditions used here, MR-visible neurochemical sensitivity is limited to compounds with tissue concentrations greater than 0.5 nmol / mg (i.e. millimolar range); therefore, these neuroimaging tools currently do not have the capacity to measure monoamines (Fowler et al., 2007).
The details for this measurement are published (Ghoddoussi et al., 2010, O'Leary-Moore et al., 2007, O'Leary-Moore et al., 2008, Perrine et al., 2008) and briefly described here. Frozen intact tissue samples, weighing ~3 mg, were placed into a 10 µL Bruker zirconium rotor containing buffer (pH = 7.4 100 mM K2HPO4/KH2PO4, 200 mM HCOONa, 1 g/L NaN3 diluted 50% with D2O containing 3 mM trimethylsilyl-propionate, which served as an internal chemical shift reference). The rotor was inserted in a Bruker magic angle spinning probe mounted in a vertical 8.9 cm-bore Bruker 11.7 T magnet (Bruker Biospin Corp., Billerica, MA). The internal temperature in the probe was maintained at 4°C and the samples were spun at 4.2 ± 0.002 kHz. The Bruker system was controlled with an Avance DRX-500 console, field inhomogeneities were compensated for using a semi-automated shimming procedure using Bruker Smart Magnet Systems, and data were acquired using Bruker-XWINNMR version 3.6 software. Following water suppression, spectra were acquired with a 1-D Carr-Purcell-Meiboom-Gill pulse sequence [90-(τ-180-τ)n-acquisition], where n=12 with recycle-time (TR) = 6210 ms, and τ = 0.15 ms for total echo-time (TE) of 3.6 ms; spectral bandwidth = 7.0 kHz (14 ppm), 16,000 complex data points, and 32 averages for total acquisition time of 3 min 48 s.
Using a basis set derived from a linear combination of 28 individual neurochemicals with a chemical shift between 1.2 – 4.2 ppm, a customized version of LCModel 6.1-4 was used to analyze spectral data (Provencher, 1993). A representative 1H-MRS spectrum from the hippocampus of an MDMA treated rat (raw data at 32 averages) as well as the full LC Model fit and LC Model fits for the NAA, INS, GABA, GLU, and GLN is shown in figure 1. Other neurochemicals of the 1H-MRS profile reported herein include cholines, creatine, glutathione, lactate and taurine. Note that (total) cholines are the sum of choline, phosphorylcholine, and glycerophosphorylcholine in order to compare our results with clinical scans that report total cholines. The precision of the LC Model fit to the spectral data was assessed with signal to noise (S/N) ratios and Cramér-Rao lower bounds. Cramér-Rao lower bounds describe the variance of estimators of a deterministic measure and, in the present experiments, they describe the precision of metabolite quantification (Cavassila et al., 2001, Tkac et al., 2009). For the hippocampus data the average ± s.e.m. for S/N was 23.6±0.5 and the average Cramér-Rao lower bounds (%) were NAA=1.3, INS=2.5, GABA=4, GLU=2, GLN=8.6 and less than 13% for all other metabolites reported herein. Spectral data were corrected for tissue weight, and concentrations are expressed as nmol neurochemical / mg tissue and shown as the mean ± s.e.m. for figures 3 and 4 and for table 1. In a single analytical session, all samples from a given brain region were analyzed in random order by a researcher blind to the experimental condition.
Figure 1. Representative 1H-MRS spectrum.
from the hippocampus of an MDMA treated Sprague-Dawley rat (raw data) as well as the full LC Model fit and LC Model fits for N-acetylaspartate (NAA), myo-inositol (INS), gamma-aminobutyric acid (GABA), glutamate (GLU), and glutamine (GLN).
Figure 3. Effects of MDMA on 1H-MRS markers of neuron and glial cell integrity.
The effects of MDMA administration on N-acetylaspartate (NAA) and myo-inositol (INS) were determined seven days after repeated injection (IP every 2 hours, 4 injections) by 1H-MRS analysis in the prefrontal cortex (A), anterior striatum (B), and hippocampus (C) of rats. Sample sizes (N) were 9 rats per group, except for prefrontal cortex NAA where N=8 for saline and MDMA, prefrontal cortex INS where saline=8, anterior striatum NAA and INS where saline=8, and hippocampus INS where saline=8. Data are shown as mean ± standard error of the mean. Data were analyzed by independent t-test (*P<0.05 and **P<0.01 for saline versus MDMA).
Figure 4. Effects of MDMA on the major excitatory and major inhibitory amino acid neurotransmitters in the brain.
The effects of MDMA administration on glutamate and GABA were determined seven days after repeated injection (IP every 2 hours, 4 injections) by 1H-MRS analysis in the prefrontal cortex (A and B), anterior striatum (C and D), and hippocampus (E and F) of rats. Sample sizes (N) were 9 rats per group, except for (A) and (B) where N=8 for saline and (E) where N=8 for saline and MDMA. Data are shown as mean ± standard error of the mean. Data were analyzed by independent t-test (*P<0.05 for saline versus MDMA).
Table 1. Effects of MDMA on neurochemicals of the 1H-Magnetic Resonance Spectroscopy profile.
Sprague Dawley rats were treated with MDMA (10 mg/kg IP, q2h ×4) or saline (1 ml/kg IP, q2h ×4) and euthanized 7 days afterwards with no treatment in the interim period. Brains were dissected and tissues analyzed using 1H-MRS. Data are shown as nanomole neurochemical / milligram tissue except for the glutamine to glutamate ratio (GLN / GLU). Sample sizes (N) were 6–9 rats per group, and data are shown as the mean ± standard error of the mean.
| Prefrontal Cortex | Anterior Striatum | Hippocampus | ||||
|---|---|---|---|---|---|---|
| saline | MDMA | saline | MDMA | saline | MDMA | |
| Alanine | 0.48±0.02 | 0.47±0.01 | 0.40±0.02 | 0.37±0.02 | 0.49±0.04 | 0.51±0.03 |
| Cholinesb | 1.52±0.31 | 1.44±0.32 | 1.58±0.14 | 1.45±0.14 | 1.65±0.07 | 1.56±0.09 |
| Creatine | 5.21±0.10 | 5.56±0.11 | 5.36±0.11 | 5.75±0.11a | 5.57±0.10 | 5.69±0.10 |
| Glutamine | 2.37±0.07 | 2.55±0.12 | 2.47±0.13 | 2.54±0.12 | 1.83±0.14 | 1.77±0.10 |
| Glutathione | 0.81±0.04 | 0.93±0.03a | 0.87±0.02 | 0.99±0.04a | 0.71±0.03 | 0.74±0.03 |
| GPCb | 0.98±0.08 | 1.06±0.04 | 0.96±0.10 | 0.94±0.10 | 0.92±0.11 | 1.27±0.06a |
| Lactate | 9.01±0.33 | 9.24±0.24 | 7.42±0.25 | 7.43±0.19 | 9.05±0.26 | 8.76±0.16 |
| Succinate | 0.34±0.02 | 0.37±0.02 | 0.29±0.02 | 0.30±0.02 | 0.28±0.01 | 0.26±0.01 |
| Taurine | 8.05±0.20 | 8.81±0.17a | 7.62±0.25 | 7.81±0.20 | 6.32±0.37 | 6.63±0.21 |
| GLN / GLU | 0.24±0.01 | 0.25±0.01 | 0.32±0.01 | 0.33±0.01 | 0.24±0.02 | 0.25±0.01 |
Data were analyzed by independent t-test (aP<0.05 for saline versus MDMA; indicated by box shaded in grey).
Note that (total) Cholines = choline + phosphorylcholine + glycerophosphorylcholine (GPC).
2.4 Statistics
Data were analyzed, graphed and tested for statistical significance using Microsoft Excel 2003, Prism 4 (Graphpad) or SPSS v16.0 software, respectively. Statistical significance was set at P<0.05 with a 95% confidence interval and independent student t-tests used to analyze control versus MDMA groups by region and neurochemical. The primary 1H-MRS related hypotheses focused on potential changes in NAA, INS and secondary hypotheses focused on potential changes in GABA and GLU as well as other compounds in the neurochemical profile.
3. Results
3.1 Effects of MDMA on monoamine neurotransmitter levels
Serotonin, dopamine and norepinephrine levels were determined by HPLC seven days after repeated MDMA injection (IP every 2 hours, 4 injections; figure 2). Serotonin levels were significantly different between saline and MDMA groups, with lower serotonin levels seen in anterior striatum (t16=5.96, P<0.0001) and hippocampus (t16=4.11, P=0.0008) of rats treated previously with MDMA (figure 2A). Similar to its effect on 5HT, MDMA decreased dopamine levels in the anterior striatum (t13=3.92, P=0.0018, figure 2B). Figure 2C shows that hippocampal norepinephrine levels were also decreased by MDMA (t16=2.54, P=0.02).
Contralateral tissue punches from anterior striatum and hippocampus were analyzed by HPLC and 1H-MRS; however, medial prefrontal cortex tissues were only analyzed by 1H-MRS due to limited sample for this area.
3.2 Effects of MDMA on N-acetylaspartate and myo-inositol
The effects of MDMA administration on NAA and INS were determined seven days after repeated injection since they may reflect effects on neuronal and glial cell integrity, respectively (figure 3). MDMA treatment significantly increased levels of NAA (t14=3.62, P=0.0028) and INS (t15=2.63, P=0.0191) in the prefrontal cortex whereas neither compound in the anterior striatum was altered by MDMA (figure 3B). In the hippocampus, 7 days after MDMA treatment, INS increased (t16=2.54, P=0.02) however NAA levels did not differ from saline treated control.
3.3 Effects of MDMA on glutamate and GABA and intermediate metabolites
The effects of MDMA on the major excitatory neurotransmitter GLU as well as on the inhibitory neurotransmitter GABA were determined seven days after MDMA treatment (figure 4). In the prefrontal cortex, GLU was significantly increased in MDMA treated rats (t16=2.43, P=0.0274, figure 4A). Repeated MDMA administration had no significant enduring effects on amino acid neurotransmitter levels in the anterior striatum when compared to saline administration. Finally, both GLU (t14=2.95, P=0.0106, figure 4E) and GABA (t16=2.13, P=0.0496, figure 4F) levels in the hippocampus were significantly decreased by prior MDMA treatment. Glutamine (GLN) levels and the ratio of glutamine to glutamate (GLN/GLU) were not affected by prior MDMA administration in any brain region (table 1).
3.4 Effects of MDMA on other neurochemicals of the 1H-MRS profile
In addition to the hypotheses tested with the neurochemicals listed above, the 1H-MRS profile yields considerably more information about the neurometabolome. These additional data are presented in Table 1. In particular, we analyzed the effect of MDMA on choline-containing compounds, creatine, glutathione, glycerophosphorylcholine (GPC), lactate and taurine. In the prefrontal cortex, prior exposure to MDMA administration increased taurine (t16=2.92, P=0.01) and glutathione (t16=2.17, P=0.0459). Glutathione (t15=2.70, P=0.0164) along with creatine (t15=2.48, P=0.0256) levels were significantly increased in the anterior striatum a week after MDMA. In the hippocampus, total cholines were not affected by prior MDMA; however, resolution of the choline peaks by HR-MAS 1H-MRS revealed a significant increase in glycerophosphorylcholine (t12=2.78, P=0.0168).
4. Discussion
4.1 Effects of MDMA on putative 1H-MRS markers of brain integrity
MDMA is a ring-substituted amphetamine thought to have neurotoxic effects akin to other amphetamine analogs like methamphetamine. Multiple studies in animals have found that repeated MDMA causes enduring disruptive effects on serotonergic activity in particular. For example, high dose MDMA depletes brain serotonin levels, a finding replicated here (figure 2A), and it reduces SERT levels in non-human primates and rodents (reviewed by (Green et al., 2003, Gudelsky and Yamamoto, 2008, Ricaurte and McCann, 2001)). In rodents, the 5HT neurotoxicity may be mediated by reactive oxygen species and reactive nitrogen species, which are elevated in the immediate period after high dose MDMA (Kuhn and Geddes, 2000, Thomas et al., 2004, Yamamoto and Raudensky, 2008). PET studies in humans also show reductions in SERT levels following MDMA use (Buchert et al., 2004, McCann et al., 1998, McCann et al., 2005); however, the neurotoxic effects of MDMA have not been easy to identify using other in vivo imaging modalities in humans.
Magnetic resonance spectroscopy, an imaging technique fundamentally similar to magnetic resonance imaging (MRI), has considerable utility for diagnostic as well as etiological neurochemistry in a number of brain diseases. One of the major peaks in the 1H-MRS chemical shift spectrum arises from the methyl protons of N-acetylaspartate (NAA). The metabolic significance of NAA is not completely understood, however its selective localization to neuronal mitochondria makes it a candidate marker of neuronal integrity, namely neuronal loss or dysfunction (Simmons et al., 1991, Urenjak et al., 1993). Capitalizing on its neuronal localization, reduced NAA has been considered a potential biomarker for several psychiatric disorders, including major depressive disorder (Sharma et al., 1992) and schizophrenia (Maier et al., 1995, Sharma et al., 1992); however, attempts to determine if NAA levels are reliable markers of brain toxicity in former MDMA users show inconsistent results.
Two 1H-MRS studies report reductions in NAA in midfrontal gray matter (Reneman et al., 2002c, Reneman et al., 2001) of former MDMA users whereas others show no change in NAA levels in hippocampus, midfrontal and midoccipital gray matter, or right parietal matter (Chang et al., 1999, Cowan et al., 2007, Daumann et al., 2004, de Win et al., 2008, de Win et al., 2007, Obergriesser et al., 2001). Given these conflicting observations, coupled with the idea that the absence of an MDMA effect on NAA does not indicate a benign response of 5HT containing neurons in humans exposed to MDMA, we sought to determine the 1H-MRS neurochemical profile in animals with MDMA-induced 5HT neurotoxicity. Preclinical animal research is scant with only one publication showing MDMA induced decreases in hypothalamic NAA levels and had no effect in frontal or occipital cortex or caudate putamen of marmoset monkeys (Meyer et al., 2006).
In attempt to clarify the human studies and to determine in a controlled setting the effects of MDMA on brain NAA levels, NAA in select brain regions was measured with the hypothesis that if 1H-MRS can detect discrete loss of 5HT containing neurons or axonal projections, then decreases in NAA would be observed. In the present study, NAA levels were unchanged in anterior striatum and hippocampus 7 days after a high dose regimen of MDMA (figure 3). In fact, prefrontal cortical NAA levels were increased in MDMA treated rats (figure 3A). Given that NAA levels (as measured by 1H-MRS) correlate tightly with measures of neuronal viability in vitro in rodent models of neuronal injury (Guimaraes et al., 1995, Strauss et al., 1997), increased NAA may represent an increase in neuronal (mitochondria-dependent) activity or density. The major finding in this study that NAA levels are not decreased under conditions of MDMA-induced 5HT depletion (figures 2A and 3A) suggests that 1H-MRS assessment of NAA levels in MDMA users may not be an accurate index of drug-induced neurotoxicity in 5HT axon terminals. It should be emphasized that the lack of effect on NAA was determined 7 days after an acute MDMA exposure whereas potential MDMA-induced damage in patients has been assessed after an extended period of repeated drug use and variable periods of abstinence. Since human studies of drug users are fraught with uncontrollable variables such as polydrug use as well as the dose and period of drug use, reported alterations in the MR-visible neurochemical profile (e.g. NAA) in MDMA-abusing humans may reflect neuroadaptations extraneous to MDMA exposure (Cowan et al., 2009). Moreover, our results suggest caution when interpreting clinical changes in NAA; specifically, the absence of an NAA effect is not evidence for the absence of neurotoxicity to specific neurons or terminal axons such as those of the serotonin system. This interpretation is strengthened by considering the ubiquitous presence of NAA and the relative density of a neuronal subtype in the total population of neuropil in a particular region of interest. In other words, the volume of 5HT axon terminals is minimal compared to the total neuronal gray matter in the region of interest; this disproportionate distribution may limit the ability to detect neurotoxicity in a specific neuronal subtype (i.e. 5HT terminals).
The clinical effects of MDMA on INS have been studied with 1H-MRS. Histological studies of inflammatory demylination and stroke show a relationship between 1H-MRS INS and glial cell activation suggesting that INS is a marker of glial cell scarring (Bitsch et al., 1999, Rumpel et al., 2003). Chang and colleagues reported increased INS in right parietal white matter of former MDMA users and suggested that this may reflect glial activation in response to serotonergic neurotoxicity (Chang et al., 1999). Besides a potential marker of glial density, INS may also reflect gliosis, microglial activation, or increased glial metabolism as a result of neurotoxicity (Cowan, 2007). In the present study INS levels were significantly increased in prefrontal cortex and hippocampus of rats 7 days following repeated MDMA administration (figures 3A & C). Although NAA was not decreased in the hippocampus, the INS increase, coupled with decreased 5HT, may indicate an early glial response to hippocampal damage (see below).
Besides its association with glial function, INS is a synthetic precursor for inositol triphosphate (IP3) and therefore plays a key role in the availability of phosphatidyl inositol and related signal transduction cascades. Disruption of signaling pathways linked to IP3 (e.g. 5HT2A receptor) may contribute to the observed increase in INS. Serotonin 2A receptors may be particularly vulnerable since they are activated by both extracellular 5HT and MDMA itself; 5HT2A receptors mediate hallucinogenesis and regulate GLU excitability in the prefrontal cortex (Lambe and Aghajanian, 2001, 2007, Lambe et al., 2000). In rats, 5HT2A receptor density is initially decreased then recovers to baseline or greater 7 days following MDMA cessation (Reneman et al., 2002b). Interestingly, a similar biphasic effect on 5HT2A receptor density is evident in MDMA users, depending on the extent of abstinence (Reneman et al., 2002b).
Human studies have shown that cholines are not changed as a result of past MDMA use (Chang et al., 1999, Obergriesser et al., 2001) and the total cholines data shown here are similar (table 1). However, the ability (at 11.7T) to resolve the choline-containing neurochemicals in the present analysis reveals increased hippocampal GPC in MDMA-exposed animals (table 1). Since GPC is produced only after phospholipase A2 hydrolysis of phosphatidylcholine as the first step in the synthesis of inflammatory mediators (e.g. leukotrienes, prostaglandins, and platelet activating factor), increased GPC may reflect a heightened inflammatory state in the hippocampus 7 days after MDMA (Aguirre et al., 1999). The anti-oxidant glutathione is also resolved by high field HR-MAS 1H-MRS and we observed an increase in the prefrontal cortex and striatum but not hippocampus, after prior MDMA exposure (table 1). The selective increases in glutathione suggest a compensatory neuroprotective response in the prefrontal cortex and anterior striatum but not hippocampus.
4.2 Effects of MDMA on neurotransmitters
As described above the effects of MDMA on monoamine neurotransmitter levels, particularly 5HT, are severe and widespread in the brain (figure 2). Since monoamines modulate the synaptic efficacy of glutamate and GABA (Sesack and Grace, 2010), it was of interest to understand how monoamine decrements affect the amino acid transmitters. At high field strength using HR-MAS 1H-MRS, GLU and GABA and the metabolite GLN are reliably measureable in intact tissue samples (figure 1).
Glutamate levels were increased in prefrontal cortex, not affected in anterior striatum, and decreased in the hippocampus of MDMA treated compared to saline treated rats (figure 4). In the most basic interpretation, changes in 1H-MRS levels of GLU (or GABA) may reflect a change in their respective nerve terminal density. Additionally, GLU levels may telegraph the magnitude of GLU neurotransmission, anaplerosis, or GLN-GLU cycling in astrocytes. Since the GLN-GLU cycle is influenced by astrocyte function (Rothman et al., 2003), the stability of the GLN/GLU ratio after MDMA treatment suggests that GLU homeostasis is maintained (table 1). Lactate, alanine, and succinate are TCA cycle precursors and intermediates, and studies have shown that 1H-MRS lactate levels reflect oxidative metabolism (Saneto et al., 2008) and 1H-MRS succinate levels are altered in mitochondrial diseases (Bianchi et al., 2007). Since prior MDMA treatment did not affect these biochemicals (table 1), mitochondrial homeostasis appears intact and changes in GLU are not likely the result of anaplerotic reactions that shunt GLU into the TCA cycle by way of α-ketoglutarate. Taken together, the data suggest that MDMA-induced changes in GLU likely reflect changes in neurotransmission or GLU neuron density. Further studies are necessary to fully understand how changes in 1H-MRS GLU levels relate to behavior and cognitive changes associated with former MDMA abuse.
GABA levels were unchanged in prefrontal cortex and anterior striatum; however, similar to the GLU response, prior exposure to MDMA reduced hippocampal GABA. Although hippocampal GABA has yet to be measured in human MDMA users, disruption of GABA innervation (figure 4) may be related to the behavioral deficits associated with MDMA abuse such as disinhibition and depression (Guillot and Berman, 2007, McCann et al., 1996, Montoya et al., 2002, Thomasius et al., 2006). For example, major depressive disorder is associated with lower brain GABA levels, and in patients with depression, the GABA deficit is reversed with selective-serotonin reuptake inhibitor (SSRI) antidepressant pharmacotherapy (Sanacora et al., 2000, Sanacora and Saricicek, 2007). As related to neurochemistry, drugs that increase GABAA receptor channel opening ameliorate the neurotoxic effect of MDMA on hippocampal 5HT, an effect that appears to be related to MDMA-induced hyperthermia (Colado et al., 2001). Lastly, as suggested by Gao (Gao et al., 2007), who reported decreased hippocampal GABA in morphine-withdrawn rats, drug-induced alterations in GABA (and GLU) may represent an adaptive metabolic response. Whether the GABA deficit noted in the present experiment represents a loss of GABA innervation or enables 5HT neurotoxicity remains to be determined.
4.3. Limitations and Summary
This study has limitations. First, although NAA and myo-inositol are often considered markers of neuronal and glial cell integrity, respectively, the physiological role of these compounds is either extensive (myo-inositol) or not clearly understood (NAA). NAA is localized to mitochondria within neurons and therefore it is a reasonable marker of neuronal density; however its role in cellular homeostasis is unknown and levels may change in response to energy demands. The use of INS as a marker of glial cell integrity and activity is based on studies using traditional histological techniques to validate the 1H-MRS findings (Bitsch et al., 1999, Rumpel et al., 2003); nonetheless, other processes that utilize INS (e.g. a substrate for PIP2, an osmolyte) conceivably could alter levels of INS. Second and similar to the first limitation, the ability of the 1H-MRS technique to detect MDMA-induced loss of 5-HT containing neurons or axonal projections is by inference from the neurochemical profiles. Thus, it is possible that neuronal function is impaired in the absence of neuronal loss. Third, MDMA-induced hyperthermia was not measured nor was core body temperature adjusted during drug treatment. Thus, potential inter-individual differences in core body temperature were not corrected or weighted in the analysis, and comparisons to other studies (with similar dosing regimens but higher or lower ambient temperature) is limited. Finally the statistical analysis does not include a correction for multiple comparisons across neurochemicals; therefore the potential for Type I error is acknowledged.
Seven days after a neurotoxic dose of MDMA, NAA levels were not reduced compared to saline treated controls suggesting that NAA, thought to be localized to neurons, may not be a suitable biomarker for loss of 5HT projections. However, 1H-MRS analysis revealed persistent alterations in other neurotransmitter systems that may relate to the negative behavioral effects of MDMA abuse. In this regard, the hippocampus shows a 1H-MRS pattern suggestive of a particular vulnerability to MDMA insult. Although NAA was unchanged, 5HT, GLU, and GABA were decreased while INS and GPC were increased in this brain region, a pattern of changes consistent with hippocampal vulnerability to MDMA (Kindlundh-Hogberg et al., 2008).
Amino acid neurotransmitters (measured by 1H-MRS) were altered in the rat medial prefrontal cortex and the hippocampus, but not anterior striatum. These neurochemical alterations may be associated with the behavioral effects of MDMA and the functional neuroanatomy governed by these brain regions. For example, levels of striatal GLU or GABA are unchanged by prior MDMA exposure, consistent with the reduced reinforcement property of MDMA (De La Garza et al., 2007). On the other hand, MDMA significantly alters GLU levels in prefrontal cortex and hippocampus and it is well established that MDMA affects cortical and hippocampal-dependent behaviors including impulsivity and long-term memory deficits (Morgan et al., 2006, Thomasius et al., 2005). The mechanism by which MDMA alters amino acid neurotransmitter levels, as well as the functional implications of these adaptations is the focus of ongoing investigations.
Acknowledgments
The authors thank Raghavendra (Raj) Nayak, M.S. for technical assistance. This research was supported by the National Institute on Drug Abuse to S.A.P. (K01-DA024760) and M.P.G. (R01-DA016736).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguirre N, Barrionuevo M, Ramirez MJ, Del Rio J, Lasheras B. Alpha-lipoic acid prevents 3,4-methylenedioxy-methamphetamine (MDMA)-induced neurotoxicity. Neuroreport. 1999;10:3675–3680. doi: 10.1097/00001756-199911260-00039. [DOI] [PubMed] [Google Scholar]
- Ball KT, Wellman CL, Fortenberry E, Rebec GV. Sensitizing regimens of (+/−)3, 4-methylenedioxymethamphetamine (ecstasy) elicit enduring and differential structural alterations in the brain motive circuit of the rat. Neuroscience. 2009;160:264–274. doi: 10.1016/j.neuroscience.2009.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglia G, Yeh SY, O'Hearn E, Molliver ME, Kuhar MJ, De Souza EB. 3,4-Methylenedioxymethamphetamine and 3,4-methylenedioxyamphetamine destroy serotonin terminals in rat brain: quantification of neurodegeneration by measurement of [3H]paroxetine-labeled serotonin uptake sites. J Pharmacol Exp Ther. 1987;242:911–916. [PubMed] [Google Scholar]
- Bianchi MC, Sgandurra G, Tosetti M, Battini R, Cioni G. Brain magnetic resonance in the diagnostic evaluation of mitochondrial encephalopathies. Biosci Rep. 2007;27:69–85. doi: 10.1007/s10540-007-9046-z. [DOI] [PubMed] [Google Scholar]
- Bitsch A, Bruhn H, Vougioukas V, Stringaris A, Lassmann H, Frahm J, et al. Inflammatory CNS demyelination: histopathologic correlation with in vivo quantitative proton MR spectroscopy. AJNR Am J Neuroradiol. 1999;20:1619–1627. [PMC free article] [PubMed] [Google Scholar]
- 6.Buchert R, Thomasius R, Wilke F, Petersen K, Nebeling B, Obrocki J, et al. A voxel-based PET investigation of the long-term effects of "Ecstasy" consumption on brain serotonin transporters. Am J Psychiatry. 2004;161:1181–1189. doi: 10.1176/appi.ajp.161.7.1181. [DOI] [PubMed] [Google Scholar]
- Camicioli RM, Hanstock CC, Bouchard TP, Gee M, Fisher NJ, Martin WR. Magnetic resonance spectroscopic evidence for presupplementary motor area neuronal dysfunction in Parkinson's disease. Mov Disord. 2007;22:382–386. doi: 10.1002/mds.21288. [DOI] [PubMed] [Google Scholar]
- Cavassila S, Deval S, Huegen C, van Ormondt D, Graveron-Demilly D. Cramer-Rao bounds: an evaluation tool for quantitation. NMR Biomed. 2001;14:278–283. doi: 10.1002/nbm.701. [DOI] [PubMed] [Google Scholar]
- Chang L, Ernst T, Grob CS, Poland RE. Cerebral (1)H MRS alterations in recreational 3, 4-methylenedioxymethamphetamine (MDMA, "ecstasy") users. J Magn Reson Imaging. 1999;10:521–526. doi: 10.1002/(sici)1522-2586(199910)10:4<521::aid-jmri4>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Colado MI, O'Shea E, Esteban B, Green AR. Studies on the neuroprotective effect of the enantiomers of AR-A008055, a compound structurally related to clomethiazole, on MDMA ("ecstasy")-induced neurodegeneration in rat brain. Psychopharmacology (Berl) 2001;157:82–88. doi: 10.1007/s002130100762. [DOI] [PubMed] [Google Scholar]
- Commins DL, Vosmer G, Virus RM, Woolverton WL, Schuster CR, Seiden LS. Biochemical and histological evidence that methylenedioxymethylamphetamine (MDMA) is toxic to neurons in the rat brain. J Pharmacol Exp Ther. 1987;241:338–345. [PubMed] [Google Scholar]
- Cowan RL. Neuroimaging research in human MDMA users: a review. Psychopharmacology (Berl) 2007;189:539–556. doi: 10.1007/s00213-006-0467-3. [DOI] [PubMed] [Google Scholar]
- Cowan RL, Bolo NR, Dietrich M, Haga E, Lukas SE, Renshaw PF. Occipital cortical proton MRS at 4 Tesla in human moderate MDMA polydrug users. Psychiatry Res. 2007;155:179–188. doi: 10.1016/j.pscychresns.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan RL, Joers JM, Dietrich MS. N-acetylaspartate (NAA) correlates inversely with cannabis use in a frontal language processing region of neocortex in MDMA (Ecstasy) polydrug users: A 3 T magnetic resonance spectroscopy study. Pharmacol Biochem Behav. 2009;92:105–110. doi: 10.1016/j.pbb.2008.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan RL, Roberts DM, Joers JM. Neuroimaging in human MDMA (Ecstasy) users. Ann N Y Acad Sci. 2008;1139:291–298. doi: 10.1196/annals.1432.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daumann J, Fischermann T, Pilatus U, Thron A, Moeller-Hartmann W, Gouzoulis-Mayfrank E. Proton magnetic resonance spectroscopy in ecstasy (MDMA) users. Neurosci Lett. 2004;362:113–116. doi: 10.1016/j.neulet.2004.03.004. [DOI] [PubMed] [Google Scholar]
- De La Garza R, 2nd, Fabrizio KR, Gupta A. Relevance of rodent models of intravenous MDMA self-administration to human MDMA consumption patterns. Psychopharmacology (Berl) 2007;189:425–434. doi: 10.1007/s00213-005-0255-5. [DOI] [PubMed] [Google Scholar]
- de Win MM, Jager G, Booij J, Reneman L, Schilt T, Lavini C, et al. Sustained effects of ecstasy on the human brain: a prospective neuroimaging study in novel users. Brain. 2008;131:2936–2945. doi: 10.1093/brain/awn255. [DOI] [PubMed] [Google Scholar]
- de Win MM, Reneman L, Jager G, Vlieger EJ, Olabarriaga SD, Lavini C, et al. A prospective cohort study on sustained effects of low-dose ecstasy use on the brain in new ecstasy users. Neuropsychopharmacology. 2007;32:458–470. doi: 10.1038/sj.npp.1301225. [DOI] [PubMed] [Google Scholar]
- Duc CO, Trabesinger AH, Weber OM, Meier D, Walder M, Wieser HG, et al. Quantitative 1H MRS in the evaluation of mesial temporal lobe epilepsy in vivo. Magn Reson Imaging. 1998;16:969–979. doi: 10.1016/s0730-725x(98)00123-4. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Ciullo JR, Wakabayashi KT, De La Garza R, 2nd, Traynor JR, Woods JH. A comparison of the physiological, behavioral, neurochemical and microglial effects of methamphetamine and 3,4-methylenedioxymethamphetamine in the mouse. Neuroscience. 2008;151:533–543. doi: 10.1016/j.neuroscience.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler JS, Volkow ND, Kassed CA, Chang L. Imaging the addicted human brain. Sci Pract Perspect. 2007;3:4–16. doi: 10.1151/spp07324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Xiang Y, Sun N, Zhu H, Wang Y, Liu M, et al. Metabolic changes in rat prefrontal cortex and hippocampus induced by chronic morphine treatment studied ex vivo by high resolution 1H NMR spectroscopy. Neurochem Int. 2007;50:386–394. doi: 10.1016/j.neuint.2006.09.012. [DOI] [PubMed] [Google Scholar]
- Ghoddoussi F, Galloway MP, Jambekar A, Bame M, Needleman R, Brusilow WS. Methionine sulfoximine, an inhibitor of glutamine synthetase, lowers brain glutamine and glutamate in a mouse model of ALS. J Neurol Sci. 2010;290:41–47. doi: 10.1016/j.jns.2009.11.013. [DOI] [PubMed] [Google Scholar]
- Green AR, Mechan AO, Elliott JM, O'Shea E, Colado MI. The pharmacology and clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA, "ecstasy") Pharmacol Rev. 2003;55:463–508. doi: 10.1124/pr.55.3.3. [DOI] [PubMed] [Google Scholar]
- Gudelsky GA, Yamamoto BK. Actions of 3,4-methylenedioxymethamphetamine (MDMA) on cerebral dopaminergic, serotonergic and cholinergic neurons. Pharmacol Biochem Behav. 2008;90:198–207. doi: 10.1016/j.pbb.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillot CR, Berman ME. MDMA (Ecstasy) use and psychiatric problems. Psychopharmacology (Berl) 2007;189:575–576. doi: 10.1007/s00213-006-0606-x. [DOI] [PubMed] [Google Scholar]
- Guimaraes AR, Schwartz P, Prakash MR, Carr CA, Berger UV, Jenkins BG, et al. Quantitative in vivo 1H nuclear magnetic resonance spectroscopic imaging of neuronal loss in rat brain. Neuroscience. 1995;69:1095–1101. doi: 10.1016/0306-4522(95)00300-8. [DOI] [PubMed] [Google Scholar]
- Hajek M, Dezortova M, Komarek V. 1H MR spectroscopy in patients with mesial temporal epilepsy. Magma. 1998;7:95–114. doi: 10.1007/BF02592234. [DOI] [PubMed] [Google Scholar]
- Kindlundh-Hogberg AM, Blomqvist A, Malki R, Schioth HB. Extensive neuroadaptive changes in cortical gene-transcript expressions of the glutamate system in response to repeated intermittent MDMA administration in adolescent rats. BMC Neurosci. 2008;9:39. doi: 10.1186/1471-2202-9-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch S, Galloway MP. MDMA induced dopamine release in vivo: role of endogenous serotonin. J Neural Transm. 1997;104:135–146. doi: 10.1007/BF01273176. [DOI] [PubMed] [Google Scholar]
- Kuhn DM, Geddes TJ. Molecular footprints of neurotoxic amphetamine action. Ann N Y Acad Sci. 2000;914:92–103. doi: 10.1111/j.1749-6632.2000.tb05187.x. [DOI] [PubMed] [Google Scholar]
- Lambe EK, Aghajanian GK. The role of Kv1.2-containing potassium channels in serotonin-induced glutamate release from thalamocortical terminals in rat frontal cortex. J Neurosci. 2001;21:9955–9963. doi: 10.1523/JNEUROSCI.21-24-09955.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambe EK, Aghajanian GK. Prefrontal cortical network activity: Opposite effects of psychedelic hallucinogens and D1/D5 dopamine receptor activation. Neuroscience. 2007;145:900–910. doi: 10.1016/j.neuroscience.2006.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambe EK, Goldman-Rakic PS, Aghajanian GK. Serotonin induces EPSCs preferentially in layer V pyramidal neurons of the frontal cortex in the rat. Cereb Cortex. 2000;10:974–980. doi: 10.1093/cercor/10.10.974. [DOI] [PubMed] [Google Scholar]
- Maier M, Ron MA, Barker GJ, Tofts PS. Proton magnetic resonance spectroscopy: an in vivo method of estimating hippocampal neuronal depletion in schizophrenia. Psychol Med. 1995;25:1201–1209. doi: 10.1017/s0033291700033171. [DOI] [PubMed] [Google Scholar]
- McCann UD, Slate SO, Ricaurte GA. Adverse reactions with 3,4-methylenedioxymethamphetamine (MDMA; 'ecstasy') Drug Saf. 1996;15:107–115. doi: 10.2165/00002018-199615020-00003. [DOI] [PubMed] [Google Scholar]
- McCann UD, Szabo Z, Scheffel U, Dannals RF, Ricaurte GA. Positron emission tomographic evidence of toxic effect of MDMA ("Ecstasy") on brain serotonin neurons in human beings. Lancet. 1998;352:1433–1437. doi: 10.1016/s0140-6736(98)04329-3. [DOI] [PubMed] [Google Scholar]
- McCann UD, Szabo Z, Seckin E, Rosenblatt P, Mathews WB, Ravert HT, et al. Quantitative PET studies of the serotonin transporter in MDMA users and controls using [11C]McN5652 and [11C]DASB. Neuropsychopharmacology. 2005;30:1741–1750. doi: 10.1038/sj.npp.1300736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes-Ribeiro JA, Soares R, Simoes-Ribeiro F, Guimaraes ML. Reduction in temporal N-acetylaspartate and creatine (or choline) ratio in temporal lobe epilepsy: does this 1H-magnetic resonance spectroscopy finding mean poor seizure control? J Neurol Neurosurg Psychiatry. 1998;65:518–522. doi: 10.1136/jnnp.65.4.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JS, Brevard ME, Piper BJ, Ali SF, Ferris CF. Neural effects of MDMA as determined by functional magnetic resonance imaging and magnetic resonance spectroscopy in awake marmoset monkeys. Ann N Y Acad Sci. 2006;1074:365–376. doi: 10.1196/annals.1369.036. [DOI] [PubMed] [Google Scholar]
- Montoya AG, Sorrentino R, Lukas SE, Price BH. Long-term neuropsychiatric consequences of "ecstasy" (MDMA): a review. Harv Rev Psychiatry. 2002;10:212–220. [PubMed] [Google Scholar]
- Moore GJ, Galloway MP. Magnetic resonance spectroscopy: neurochemistry and treatment effects in affective disorders. Psychopharmacol Bull. 2002;36:5–23. [PubMed] [Google Scholar]
- Morgan MJ, Impallomeni LC, Pirona A, Rogers RD. Elevated impulsivity and impaired decision-making in abstinent Ecstasy (MDMA) users compared to polydrug and drug-naive controls. Neuropsychopharmacology. 2006;31:1562–1573. doi: 10.1038/sj.npp.1300953. [DOI] [PubMed] [Google Scholar]
- O'Hearn E, Battaglia G, De Souza EB, Kuhar MJ, Molliver ME. Methylenedioxyamphetamine (MDA) and methylenedioxymethamphetamine (MDMA) cause selective ablation of serotonergic axon terminals in forebrain: immunocytochemical evidence for neurotoxicity. J Neurosci. 1988;8:2788–2803. doi: 10.1523/JNEUROSCI.08-08-02788.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Leary-Moore SK, Galloway MP, McMechan AP, Hannigan JH, Bowen SE. Region-dependent alterations in glutamate and GABA measured by high-resolution magnetic resonance spectroscopy following acute binge inhalation of toluene in juvenile rats. Neurotoxicol Teratol. 2007 doi: 10.1016/j.ntt.2007.03.062. [DOI] [PubMed] [Google Scholar]
- O'Leary-Moore SK, McMechan AP, Galloway MP, Hannigan JH. Neonatal alcohol-induced region-dependent changes in rat brain neurochemistry measured by high-resolution magnetic resonance spectroscopy. Alcohol Clin Exp Res. 2008;32:1697–1707. doi: 10.1111/j.1530-0277.2008.00747.x. [DOI] [PubMed] [Google Scholar]
- Obergriesser T, Ende G, Braus DF, Henn FA. Hippocampal 1H-MRSI in ecstasy users. Eur Arch Psychiatry Clin Neurosci. 2001;251:114–116. doi: 10.1007/s004060170044. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 6 ed. London: Academic Press; 2006. [Google Scholar]
- Perrine SA, Michaels MS, Ghoddoussi F, Hyde EM, Tancer ME, Galloway MP. Cardiac effects of MDMA on the metabolic profile determined with (1)H-magnetic resonance spectroscopy in the rat. NMR Biomed. 2008 doi: 10.1002/nbm.1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30:672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- Reneman L, Booij J, Habraken JB, De Bruin K, Hatzidimitriou G, Den Heeten GJ, et al. Validity of [123I]beta-CIT SPECT in detecting MDMA-induced serotonergic neurotoxicity. Synapse. 2002a;46:199–205. doi: 10.1002/syn.10130. [DOI] [PubMed] [Google Scholar]
- Reneman L, Endert E, de Bruin K, Lavalaye J, Feenstra MG, de Wolff FA, et al. The acute and chronic effects of MDMA ("ecstasy") on cortical 5-HT2A receptors in rat and human brain. Neuropsychopharmacology. 2002b;26:387–396. doi: 10.1016/S0893-133X(01)00366-9. [DOI] [PubMed] [Google Scholar]
- Reneman L, Majoie CB, Flick H, den Heeten GJ. Reduced N-acetylaspartate levels in the frontal cortex of 3,4-methylenedioxymethamphetamine (Ecstasy) users: preliminary results. AJNR Am J Neuroradiol. 2002c;23:231–237. [PMC free article] [PubMed] [Google Scholar]
- Reneman L, Majoie CB, Schmand B, van den Brink W, den Heeten GJ. Prefrontal N-acetylaspartate is strongly associated with memory performance in (abstinent) ecstasy users: preliminary report. Biol Psychiatry. 2001;50:550–554. doi: 10.1016/s0006-3223(01)01177-5. [DOI] [PubMed] [Google Scholar]
- Ricaurte GA, DeLanney LE, Irwin I, Langston JW. Toxic effects of MDMA on central serotonergic neurons in the primate: importance of route and frequency of drug administration. Brain Res. 1988;446:165–168. doi: 10.1016/0006-8993(88)91309-1. [DOI] [PubMed] [Google Scholar]
- Ricaurte GA, McCann UD. Experimental studies on 3,4-methylenedioxymethamphetamine (MDA, "ecstasy") and its potential to damage brain serotonin neurons. Neurotox Res. 2001;3:85–99. doi: 10.1007/BF03033232. [DOI] [PubMed] [Google Scholar]
- Rothman DL, Behar KL, Hyder F, Shulman RG. In vivo NMR studies of the glutamate neurotransmitter flux and neuroenergetics: implications for brain function. Annu Rev Physiol. 2003;65:401–427. doi: 10.1146/annurev.physiol.65.092101.142131. [DOI] [PubMed] [Google Scholar]
- Rumpel H, Lim WE, Chang HM, Chan LL, Ho GL, Wong MC, et al. Is myo-inositol a measure of glial swelling after stroke? A magnetic resonance study. J Magn Reson Imaging. 2003;17:11–19. doi: 10.1002/jmri.10233. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Mason GF, Krystal JH. Impairment of GABAergic transmission in depression: new insights from neuroimaging studies. Crit Rev Neurobiol. 2000;14:23–45. doi: 10.1615/critrevneurobiol.v14.i1.20. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Saricicek A. GABAergic contributions to the pathophysiology of depression and the mechanism of antidepressant action. CNS Neurol Disord Drug Targets. 2007;6:127–140. doi: 10.2174/187152707780363294. [DOI] [PubMed] [Google Scholar]
- Saneto RP, Friedman SD, Shaw DW. Neuroimaging of mitochondrial disease. Mitochondrion. 2008;8:396–413. doi: 10.1016/j.mito.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffel U, Szabo Z, Mathews WB, Finley PA, Dannals RF, Ravert HT, et al. In vivo detection of short- and long-term MDMA neurotoxicity--a positron emission tomography study in the living baboon brain. Synapse. 1998;29:183–192. doi: 10.1002/(SICI)1098-2396(199806)29:2<183::AID-SYN9>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Schmued LC. Demonstration and localization of neuronal degeneration in the rat forebrain following a single exposure to MDMA. Brain Res. 2003;974:127–133. doi: 10.1016/s0006-8993(03)02563-0. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Grace AA. Cortico-Basal Ganglia reward network: microcircuitry. Neuropsychopharmacology. 2010;35:27–47. doi: 10.1038/npp.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R, Venkatasubramanian PN, Barany M, Davis JM. Proton magnetic resonance spectroscopy of the brain in schizophrenic and affective patients. Schizophr Res. 1992;8:43–49. doi: 10.1016/0920-9964(92)90059-e. [DOI] [PubMed] [Google Scholar]
- Shulman RG, Blamire AM, Rothman DL, McCarthy G. Nuclear magnetic resonance imaging and spectroscopy of human brain function. Proc Natl Acad Sci U S A. 1993;90:3127–3133. doi: 10.1073/pnas.90.8.3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons ML, Frondoza CG, Coyle JT. Immunocytochemical localization of N-acetylaspartate with monoclonal antibodies. Neuroscience. 1991;45:37–45. doi: 10.1016/0306-4522(91)90101-s. [DOI] [PubMed] [Google Scholar]
- Slikker W, Jr, Ali SF, Scallet AC, Frith CH, Newport GD, Bailey JR. Neurochemical and neurohistological alterations in the rat and monkey produced by orally administered methylenedioxymethamphetamine (MDMA) Toxicol Appl Pharmacol. 1988;94:448–457. doi: 10.1016/0041-008x(88)90285-2. [DOI] [PubMed] [Google Scholar]
- Strauss I, Williamson JM, Bertram EH, Lothman EW, Fernandez EJ. Histological and 1H magnetic resonance spectroscopic imaging analysis of quinolinic acid-induced damage to the rat striatum. Magn Reson Med. 1997;37:24–33. doi: 10.1002/mrm.1910370106. [DOI] [PubMed] [Google Scholar]
- Thomas DM, Dowgiert J, Geddes TJ, Francescutti-Verbeem D, Liu X, Kuhn DM. Microglial activation is a pharmacologically specific marker for the neurotoxic amphetamines. Neurosci Lett. 2004;367:349–354. doi: 10.1016/j.neulet.2004.06.065. [DOI] [PubMed] [Google Scholar]
- Thomasius R, Petersen KU, Zapletalova P, Wartberg L, Zeichner D, Schmoldt A. Mental disorders in current and former heavy ecstasy (MDMA) users. Addiction. 2005;100:1310–1319. doi: 10.1111/j.1360-0443.2005.01180.x. [DOI] [PubMed] [Google Scholar]
- Thomasius R, Zapletalova P, Petersen K, Buchert R, Andresen B, Wartberg L, et al. Mood, cognition and serotonin transporter availability in current and former ecstasy (MDMA) users: the longitudinal perspective. J Psychopharmacol. 2006;20:211–225. doi: 10.1177/0269881106059486. [DOI] [PubMed] [Google Scholar]
- Tkac I, Oz G, Adriany G, Ugurbil K, Gruetter R. In vivo 1H NMR spectroscopy of the human brain at high magnetic fields: metabolite quantification at 4T vs. 7T. Magn Reson Med. 2009;62:868–879. doi: 10.1002/mrm.22086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urenjak J, Williams SR, Gadian DG, Noble M. Proton nuclear magnetic resonance spectroscopy unambiguously identifies different neural cell types. J Neurosci. 1993;13:981–989. doi: 10.1523/JNEUROSCI.13-03-00981.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Baumann MH, Xu H, Rothman RB. 3,4-methylenedioxymethamphetamine (MDMA) administration to rats decreases brain tissue serotonin but not serotonin transporter protein and glial fibrillary acidic protein. Synapse. 2004;53:240–248. doi: 10.1002/syn.20058. [DOI] [PubMed] [Google Scholar]
- Yamamoto BK, Raudensky J. The role of oxidative stress, metabolic compromise, and inflammation in neuronal injury produced by amphetamine-related drugs of abuse. J Neuroimmune Pharmacol. 2008;3:203–217. doi: 10.1007/s11481-008-9121-7. [DOI] [PMC free article] [PubMed] [Google Scholar]