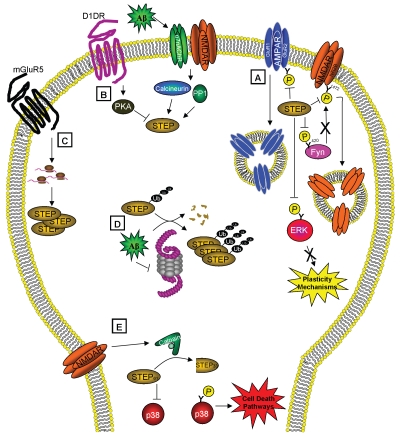
Figure 1.
STEP functions and regulation. (A) STEP activity opposes the development of synaptic plasticity by dephosphorylating and inactivating ERK and Fyn and leading to the endocytosis of AMPA and NMDA receptors. STEP is itself regulated by (B) phosphorylation, (C) local translation, (D) ubiquitination and degradation and (E) proteolytic cleavage. (B) STEP is inactivated by PKA-phosphorylation after dopamine stimulation and activated by calcineurin/PP1 dephosphorylation of this residue after α7nAChR and NMDAR stimulation. (C) Stimulation of mGluR5 leads to local translation of STEP. (D) STEP is normally degraded by the ubiquitin proteasome system (UPS), but in the presence of Aβ, the UPS is impaired, causing the accumulation of polyubiquitinated STEP. (E) Activation of extrasynaptic NMDARs triggers calpain to cleave STEP61 into STEP33, which prevents STEP from regulating its substrates and leads to the release of p38 from inhibition and activation of cell death pathways. These regulatory pathways may contribute to the pathophysiology of Alzheimer's if disrupted, and also implicate STEP as an excellent target for drug discovery.