Abstract
Diagnosis and treatment of Alzheimer’s disease (AD) depend on clinical evaluation and there is a strong need for an objective tool as a biomarker. Our group has investigated brain oscillatory responses in a small group of AD subjects. We found that the de novo (untreated) AD group differs from both the cholinergically-treated AD group and aged-matched healthy controls in theta and delta responses over left frontal-central areas after cognitive stimulation. On the contrary, the difference observed in AD groups upon a sensory visual stimulation includes response increase over primary or secondary visual sensorial areas compared to controls. These findings imply at least two different neural networks, depending on type of stimulation (i.e. cognitive or sensory). The default mode defined as activity in resting state in AD seems to be affected electrophysiologically. Coherences are also very valuable in observing the group differences, especially when a cognitive stimulus is applied. In healthy controls, higher coherence values are elicited after a cognitive stimulus than after a sensory task. Our findings support the notion of disconnectivity of cortico-cortical connections in AD. The differences in comparison of oscillatory responses upon sensory and cognitive stimulations and their role as a biomarker in AD await further investigation in series with a greater number of subjects.
Keywords: Oscillations, Event related, Alzheimer, Dementia, P300
Introduction
Alzheimer’s disease (AD) is one of the most devastating neurodegenerative diseases of elderly populations (Ferri et al. 2005). The pathology affects the temporolimbic area first and then extends to parietal and frontal lobes with relatively preserved primary motor and primary sensory areas (Braak et al. 1993). Even though many factors can influence its clinical presentation, including gender, education or socio-economical background, (Keskinoğlu et al. 2006), its diagnosis or treatment monitoring mainly depends on clinical interpretation. Therefore, there is a strong need to develop objective tools for management of this disease.
Although analysis of oscillatory processes gained tremendous importance in recent years, most of the work has focused on the analysis of spontaneous EEG oscillations. The major disturbances of EEG in AD can be summarized as posterior slowing of the EEG, reduced synchrony and complexity of the EEG (Dauwels et al. 2010). There are successful approaches indicating that EEG may serve as a biomarker for diagnosis of mild cognitive impairment and possibly also in AD (Yener et al. 1996; Babiloni et al. 2004, 2006a). Moreover, as reviewed by Başar and Güntekin (2008), the trend of analyzing cognitive neurodynamics is fast increasing in clinical applications, including the effects of transmitters. The brain dynamics in neuropsychiatric disorders (Herrmann and Demiralp 2005; Başar-Eroğlu et al. 2008; Özerdem et al. 2008a, b; Özerdem et al. 2010) can be investigated by either a linear approaches as it will be mentioned in the present review or by non-linear approaches (Jelles et al. 1999; Jeong 2004) that will not be focus of the present paper.
In healthy subjects genetics also contributes to brain responses, since the theta band energy seen in P300 experiments is related to cholinergic receptor gene (Jones et al. 2006), and human nicotinic receptor gene modulates auditory and visual event related potentials (Espeseth et al. 2007).
Event related synchronization (ERS) analysis is elicited by recording EEG during a mental task. Mild cognitive disorder (MCI) is defined as subjective memory complaint with an objective decrease in memory tests with no loss in daily activities. It has a higher conversion rate to dementia. When two subgroups of MCI (i.e. the progressive group and the stable group) were compared; the progressive group which is considered as pre-dementia state had lower theta ERS. Also cognitive decline and theta ERS showed a relationship (Missonier et al. 2006).
Similar to previous findings by Yener et al. 2009, showing higher occipital sensory visual evoked oscillation responses in AD, Osipova et al. (2006) reported increased responses of magnetoencephalography after auditory steady state 40 Hz sensory stimulation over sensory cortex, i.e. temporal lobe, in AD. As goal directed functioning requires a balance between inhibitory or excitatory inputs in the cortex, irrelevant repetitive stimulation with less cognitive load should yield to lower oscillatory responses. However, decreased inhibition of cortical auditory or visual processing, possibly due to decreased prefrontal activity, may lead to increased sensory evoked cortical responses in AD.
Several ensembles of resting-EEG oscillatory response measures were suggested on individual basis as a possible biomarker, however the automated results seemed to be less sensitive than clinicians’ rates (Dauwels et al. 2010; Polikar et al. 2007) or as good as the clinical assessment (Rossini et al. 2008). de Haan et al. (2008) reported that temporo-parietal regions showed greater difference between healthy elderly and AD subjects. The beta band, especially in the right occipital area had the greatest sensitivity (94%) and specificity (78%) in quantitative relative power analysis of magnetoencephalography recordings.
Another oscillatory dynamics study on early visual processing indicated topographic differences that distingish between healthy elderly, MCI and mild AD subjects during early visual processing (Haupt et al. 2008).
Even though involvement of the visual cortex is not well recognized in AD, it has been reported in the later stages of the disease (Braak et al. 1993; Hof and Morrison 1990; Lewis et al. 1987). More recent clinical reports, indicating either abnormalities in visuospatial abilities and visual memory (Monacelli et al. 2003; Kawas et al. 2003) or functional neuroimaging studies (Buckner et al. 2005; Drzezga et al. 2003), suggest that posterior cortical areas subserving visual processing are affected early in the course of disease. In a neuropathological study on healthy elderly, MCI and AD subjects, the visual association area was found to show dense neurofibrillary tangles (McKee et al. 2006). Most probably, cortical areas under heavy cognitive load, such as those involved in secondary associative processing, are more vulnerable to AD pathology than primary sensory areas (Morrison et al. 1987), since these regions need to develop highly adaptive and structurally dynamic connections (Stepanyants et al. 2002). Also, the primary visual cortex has generally been regarded as relatively preserved in AD, yet it shows decreased numbers of cholinergic fibers (Beach and McGeer 1992) and less enzyme activity in this area (Beelke and Sannita 2002; Davis et al. 1999). Pathological degenerative markers, including neurofibrillary tangles (NFTs) and amyloid plaques, also occur in the primary visual cortex (Morrison et al. 1991). Some visuospatial tasks have activated occipital cortex (Zeki et al. 1991), parieto-occipital junction (Tsao et al. 2003) in controls, but revealed hypoactivation in AD subjects in these regions. However, a hyperactivation was seen in alternate networks (Prvulovic et al. 2002; Stern et al. 2000), presumably due to a discontinuation of prefrontal modulation (Bentley et al. 2008; Yener et al. 2009) or to a compensatory strategy (Pariente et al. 2005).
In this short review we will focus on the neurodynamics and cognitive response oscillations in AD and encompassed comparative studies on healthy elderly controls, de novo or medicated AD subjects. We will have a special emphasis on the results of our groups on visual evoked and visual event related oscillatory responses or coherences, in two groups of mild AD and healthy elderly subjects.
Results on oscillatory brain dynamics in Alzheimer’s disease
Short state of electrophysiology
To date, many signal-processing techniques were utilized in order to reveal pathological changes in spontaneous EEG associated with AD (Jeong 2004). Theta activity in the spontaneous EEG had been reported to be different in AD than controls (Yener et al. 1996). Furthermore, spontaneous EEG has been suggested as a useful predictive technique in patients with mild cognitive impairment who will later develop AD (Cichocki et al. 2005 Rossini et al. 2006; Buscema et al. 2010). The use of quantitative evaluation of only certain periods of spontaneous EEG poses some limitations in reflecting responses in different frequency bands. Temporal summation of EEG responses after stimulation is different in AD than controls (Polich and Herbst 2000). The search for the functions of brain oscillations is now an important trend in neuroscience, since oscillatory activity in various frequency bands may reflect different aspects of information processing (Başar 1980, 2004; Karakaş et al. 2000; Buzsaki 2006). It is now clear that even the simplest cognitive functions involve large-scale neural networks, and most of the results gained after most of the results obtained from EEG or derived techniques attempt to describe the related networks (Başar 1999). Coherence (Gardner 1992) or phase-locking statistics (Maltseva et al. 2000; Lachaux et al. 2002; Bruns 2004) are some of the common techniques used to evaluate those relationships. The number of reports is rapidly increasing, as presented in a recent review on brain oscillations in cognitive impairment (Başar and Güntekin 2008). Electroencephalography and event related potentials are proposed by several authors as biomarkers in Alzheimer’s disease (For a review see, Jackson and Snyder 2008). A number of studies have been published related to the analysis of oscillatory dynamics in mild cognitive impairment (MCI) and AD patients, and several groups (Babiloni et al. 2006a, 2007, 2009) have published core results on EEG rhythms in MCI patients. Zheng-yan (2005), Hogan et al. (2003), Güntekin et al. (2008), Yener et al. (2007, 2008, 2009) and Dauwels et al. (2009) published results on Alzheimer patients. Our research group and other groups on the topic of neuropsychiatric disorders initiated several reports on cognitive processes in schizophrenia (Başar-Eroglu et al. 2009) and in two different states of bipolar disorders (Özerdem et al. 2008a, b). The publications mentioned primarily aimed to show the changes in oscillatory behavior in the above-mentioned diseases. However a more important goal of these series in clinical applications is the enrichment of the general knowledge of the nature of cognitive processes by observation of electrophysiological failures, which, in turn, may elucidate the normal functionality.
Definition and preliminary results on oscillatory dynamics in AD
Resting, eyes-closed EEG data were recorded in MCI and 65 AD subjects by Babiloni et al. (2006a). The EEG cortical sources were estimated using low-resolution brain electromagnetic tomography (LORETA). Cortical EEG sources were correlated with MR-based measurements of the lobar brain volume (white and gray matter). A negative correlation was observed between the frontal white matter and the amplitude of the frontal delta sources across the mild cognitive impairment (MCI) and AD subjects. Babiloni et al. (2007) tested the hypothesis that EEG rhythms are correlated with memory and attention in the continuum from MCI through to AD. Their results suggested that the cortical sources of resting delta and alpha rhythms correlate with neuropsychological measures of immediate memory. A recent study by Babiloni et al. (2009) indicated an information flux in the direction of parietal to frontal. Also, EEG functional coupling for alpha and beta rhythms was stronger in normal elderly than in MCI and/or AD patients (Karrasch et al. 2006).
Hogan et al. (2003) examined memory-related EEG power and coherence over temporal and central recording sites in patients with early AD and a normal control group. While the behavioral performance of patients with very mild AD did not differ significantly from that of normal controls, the AD patients had comparatively reduced upper alpha coherence between the central and right temporal cortex.
Zheng-yan (2005) stated that, during photic stimulation, the inter- and intra-hemispheric EEG coherences of AD patients were at lower values in the alpha (9.5–10.5 Hz) band than those of the control group. The author reported that, during a 5 Hz photic stimulation, the AD patients had significantly lower values of intra-hemispheric coherence in right centro-parietal and left centro-occipital electrode pairs for theta band; in the left centro-parietal, left centro-occipital and right temporo-occipital electrode pairs for alpha band; and both sides of parieto-occipital and of centro-occipital and right temporo-occipital electrode pairs for beta band oscillations.
The results presented by Missonnier et al. (2006) indicate that a decrease in the early phasic theta event related oscillation’s power during working memory activation may predict cognitive decline in MCI. This phenomenon is not related to working memory load, but may reflect the presence of early deficits in directed, attention-related neural circuits in patients with MCI.
According to the few published event related oscillation studies, it can be concluded that the left frontal and central areas in AD patients are those regions of the brain that show the most evidence of effects associated with AD (Yener et al. 2007, 2008). In these studies, it was clearly demonstrated that the most-affected frequency-bands upon the application of the oddball paradigms were in central areas at the delta and theta bands (Yener et al. 2008). Lower values of phase locking were observed in the theta band in the left frontal area (Yener et al. 2007). The relevance of these findings is that the most fundamental components of the oddball paradigms are delta and theta responses. Karrasch et al. (2006) reported in their ERD/ERS study that both AD and control groups showed event-related synchronization during retrieval period in theta frequency band (5–7 Hz), i.e. a frequency range which is commonly considered to be related to working memory, and surprisingly do not differ between the groups, whereas significant differences have been noted in the alpha (7–17 Hz) range over frontal, central and left temporal electrodes in event-related synchronization in AD (Karrasch et al. 2006).
It is also important to emphasize the effects of medication on event related oscillations in AD patients. Drugs have been reported to have local effects on theta phase synchrony in the left frontal areas and to have long-range connection effects on the alpha evoked coherence in the left fronto-parietal electrode pairs (Yener et al. 2007; Güntekin et al. 2008).
Güntekin et al. (2008) investigated event-related coherence of patients with AD forms of dementia using a visual oddball paradigm. A total of 21 mild, probable AD subjects were compared with a group of 19 healthy controls. The AD group was divided into the untreated (n = 10) and those treated with a cholinesterase inhibitor (n = 11). The authors found that the control group showed higher values of evoked coherence in the “delta”, “theta” and “alpha” bands in the left fronto-parietal electrode pairs compared to the untreated AD group. The healthy subjects showed higher values of event related coherence in the left fronto-parietal electrode pair in the theta frequency band and higher values of event related coherence in the right fronto-parietal electrode pair in the delta band when compared to the treated AD group.
Scope of our research group in the analysis of AD electrophysiology
As mentioned previously, here we included analyses of phase locking, changes in amplitudes of oscillatory responses and coherences in two groups of AD subjects and healthy elderly controls.
Phase locking
Phase locking is a manifestation of synchronization between individual neurons of neural populations upon application of a sensory or cognitive stimulation. The sensory or cognitive inputs can originate from external physical signals or can be also triggered from internal sources. Several publications report phase locking of theta oscillatory responses as a result of cognitive load in P300 target paradigm (Başar Eraglu et al. 1992; Demiralp et al. 1994; Klimesch et al. 2004). Some authors discuss the occurrence of coherence as a consequence of links between fronto-parieto-hippocampal circuitry during cognitive performance (Klimesch et al. 2008; Demiralp et al. 1994; Kahana et al. 1999; Buzsaki 2002).
Healthy subjects show strong theta phase locking in prefrontal area in P300 paradigm (Fig. 1). The principle of superposition describes integration over the temporal axis, consisting of a relationship between the amplitude and phases of oscillations in various frequency bands. Furthermore, selectively distributed and selective coherent oscillatory activities in neural populations describe integration over the spatial axis (Başar 1980). Consequently, integrative activity is a function of the coherences between spatial locations of the brain; these coherences vary according to the type of sensory and/or cognitive event and possibly the state of consciousness of the species (Başar 1999, 2004). The publications of Bressler and Kelso (2001) emphasize that the coordinated large-scale cortical network, in which the participating sites are much more interrelated to one another than to non-network sites. These coordinated areas undergo re-entrant processing, and later re-entrant interactions will constrain the local spatial activity patterns in these areas. In this manner, re-entrant transmissions define local expression of information. As areas interact reciprocally, some areas reach a consensus through the process of large-scale relative coordination, in which those areas temporarily manifest consistent local spatial activity patterns. This mechanism also provides a dynamic creation of local context in a highly adaptive manner in visual functions.
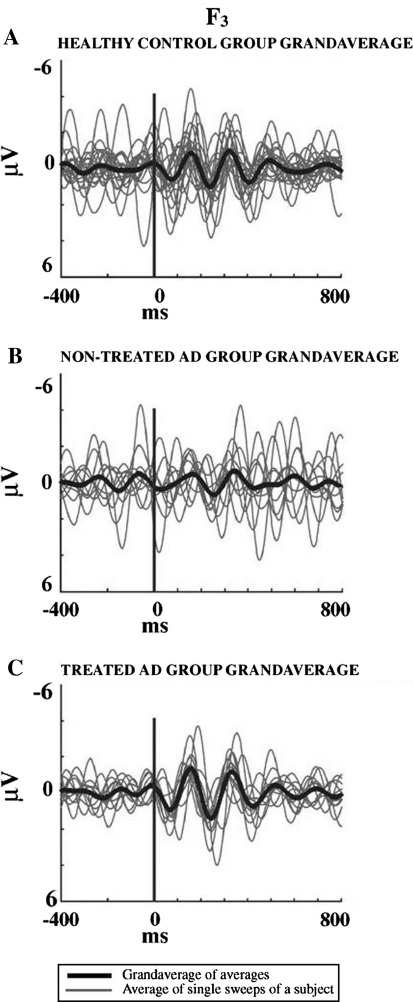
Fig. 1.
Grand-averages of theta oscillatory response healthy elderly controls and two groups of Alzheimer subjects; treated and non-treated
Varela et al. (2001) state that the emergence of a unified cognitive moment depends on the coordination of scattered parts of functionally specialized brain regions. The mechanisms of large-scale integration enable the emergence of coherent behavior and cognition (Varela et al. 2002). These authors argue that the most plausible candidate is the formation of dynamic links mediated by synchrony over multiple frequency bands. Von Stein and Sarnthein (2000) propose that long-range fronto-parietal interactions during working memory retention and mental imagery evolve instead in the theta and alpha (4–8 Hz, 8–12 Hz) frequency ranges. This large scale integration is performed by synchronization among neurons and neuronal assemblies evolving in different frequency ranges.
Yener et al. (2007) described phase locking of event-related oscillations in a pilot study involving patients with Alzheimer-type dementia (AD). Theta oscillatory responses of 22 mild probable AD subjects (11 non-treated, 11 treated by cholinesterase inhibitors) and 20 healthy elderly controls were analyzed using the conventional visual oddball paradigm. They compared theta responses of the three groups within the range 4–7 Hz at the frontal electrodes. At F3 location, theta responses of healthy subjects were phase locked to stimulation and theta oscillatory responses of non-treated Alzheimer patients showed weaker phase-locking, i.e. average of Z-transformed means of correlation coefficients between single trials was closer to zero. In treated AD patients, phase-locking following target stimulation was two times higher in comparison to the responses of non-treated patients. Their results indicated that the phase-locking of theta oscillations at F3 in the treated patients is as strong as in the control subjects. The F4 theta oscillations were not significantly different between the groups. The findings implied that the theta responses at F3 location are highly unstable in comparison to F4 in non-treated mild AD patients, and that cholinergic agents may modulate event-related theta oscillatory activities in the frontal regions. The responses shown in Fig. 1 were elicited.
These responses were elicited upon target stimuli in a classical visual oddball paradigm from the scalp electrode of F3. The thick lines indicate the grand-average of each group; the thin grey lines show averages of single sweeps from each subject in: A. The healthy elderly group (n = 20); B. The non-treated Alzheimer group (AD) (n = 11); C. The treated (cholinesterase inhibitor) Alzheimer group (n = 11).
Amplitude analysis of evoked and event related oscillations
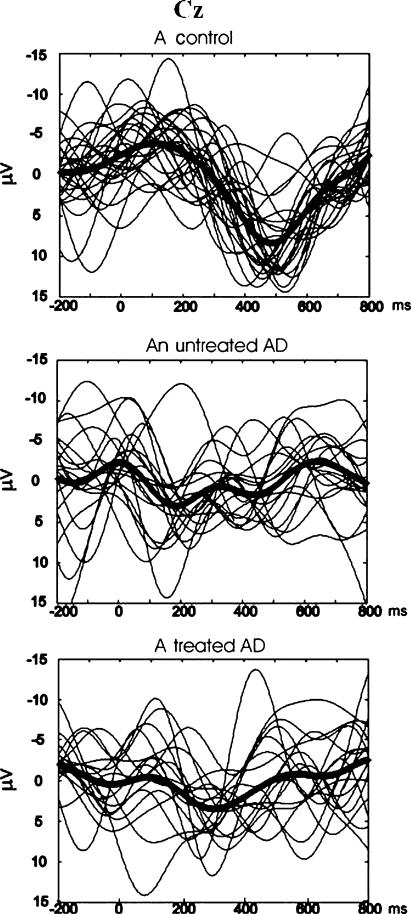
Amplitude analysis of digitally filtered evoked or event related responses provides the opportunity to explore sensory or cognitive neurodynamics. Event-related oscillations (ERO) also provide a useful tool for detecting subtle abnormalities of cognitive processes with high temporal resolution. Yener et al. (2008) analyzed event-related oscillations of patients with AD using a visual oddball paradigm. The AD group consisted of untreated patients and patients treated with a cholinesterase inhibitor. Significant differences in delta frequency range were seen between the groups at mid- and left central regions, (Cz, C3). The peak-to-peak amplitudes of delta responses of healthy subjects were significantly higher than either of the two groups of AD patients (Figs. 2, 3). For the methodological issues, the reader is referred to reviews by Başar et al. (2010) and by Güntekin and Başar (2010). Similar to these findings, by means of auditory oscillatory responses Caravaglios et al. (2008) found significant enhancement in delta responses in healthy controls when compared to Alzheimer’s subjects (especially at frontal locations). The lack of frontal delta responses regardless of stimulus modality implies a decision making impairment and decreased frontal functioning in mild AD. As shown by Vialatte et al. (2009), oscillatory responses to steady state visual flickering stimulation have a significantly higher degree of co-occurrence during these stimuli, uncorrelated with ongoing signal synchrony. The oscillatory responses undergo a consistent reorganization during visual stimulation. These findings show paralel to our group’s findings on simple sensory visual evoked oscillations in AD. In healthy adults during a conscious visuospatial task, increased prestimulus low-band alpha and decreased poststimulus high-band alpha power were observed (Babiloni et al. 2006b).
Fig. 2.
The average delta oscillatory responses (thick lines) as superimposed single trials (thinner lines) in representative subjects from each group; i.e. a healthy elderly subject, untreated AD subject, and treated AD subject
Fig. 3.
Grand averages of delta oscillatory responses elicited upon visual target stimuli from each group; i.e. healthy elderly controls (n = 20), untreated AD group (n = 11), and treated AD group (n = 11)
Cholinesterase inhibitors (ChEI) are widely used medication for the treatment of AD. They show cholinergic effects. Although ChEI have positive effects on clinical measures and spontaneous EEG parameters, their influence on brain oscillatory responses have not been explored extensively. Yener et al. (2008) reported that ChEI did not change delta oscillatory responses, but showed increased theta phase locking over frontal region (Yener et al. 2007). These studies provided further evidence for the importance of oscillatory event-related potentials in investigating brain dynamics in AD.
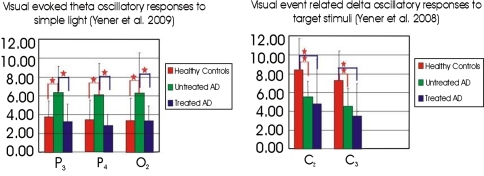
Yener et al. (2009) compared visual sensory evoked oscillatory responses of subjects with Alzheimer’s disease (AD) to those of healthy elderly controls elicited by simple light stimuli. The visual evoked oscillatory responses in AD subjects without cholinergic treatment showed significant differences from the controls and the AD subjects treated with a cholinesterase inhibitor. Higher theta oscillatory responses in untreated AD subjects were seen on the electrode locations over bi-parietal and right occipital regions after simple light stimuli with less, if any, cognitive load. These changes were restricted to the theta frequency range only and were related to location, frequency bands and drug effects. The authors observed that visual event related oscillations elicited after the visual stimuli with a higher cognitive load (i.e. an oddball target) and displayed lower amplitudes in the delta frequency band among AD subjects, regardless of drug effect, over the left and mid-central regions. These differences between the visual evoked oscillations and the visual event related oscillations implied that there were at least two different cognitive circuits that were activated upon visual stimuli in AD patients (Fig. 4).
Fig. 4.
Comparison of topology or changes of amplitudes in subject groups after sensory evoked and event related oscillatory responses indicate differential circuitry, depending on the type of stimuli, i.e. sensory or cognitive. Asterisks indicate significance at the level of 0.01
Comparison of sensory evoked and event related coherences
Sensory evoked coherences reflect the property of sensory networks activated by a sensory stimulation. Event related (or cognitive) coherences manifest coherent activity of sensory and cognitive networks triggered by a cognitive task. Accordingly, the cognitive response coherences comprehend activation of a greater number of neural networks that are most likely not activated or less activated in the EEG and sensory evoked coherences. Therefore, event related coherences merit special attention. In AD patients with strong cognitive impairment, it is particularly relevant to analyze whether medical treatment (drug application) acts selectively upon sensory and cognitive networks manifested in topologically different places and in different frequency windows. Such an observation may serve, in future, to provide a deeper understanding in physiology of distributed functional networks and, in turn, the possibility of determining biomarkers for medical treatment.
According to Başar (2006), the coherence—i.e. the connectivity between brain structures—is selective, depending on cognitive load. This is manifested in the selective distribution of the coherence functions over various brain structures with varying degrees of coherence between 0 and 1. Demonstration of the principle of selective connectivity requires the analysis of oscillations in several neural populations and in several frequency windows. Such analyses and the related findings have been fundamental to the refinement of the concepts of “whole brain” and “cooperation”.
Many studies reported the successful use of EEG coherence to measure functional connectivity (Lopes Da Silva et al. 1980; Petsche and Etlinger 1998; Rappelsberger and Pockberger 1987). According to these studies, EEG coherence may be considered to be an indispensable large-scale measure of functional relationships between pairs of cortical regions (Nunez 1997). In epileptic patients intracranial recordings reveal increased synchronization in the beta 2 frequency band related to the cognitive activity (Kukleta et al. 2009). However, it is hard to conclude whether this finding is related to normal physiology or epileptic changes seen in the mentioned subkect group. It is also important to mention the studies of T.H. Bullock’s research group. Bullock et al. (1995) clearly showed that the connectivity (coherence) between neural groups is a main factor for the evolution of cognitive processes. According to Bullock and Basar (1988) and Bullock et al. (1995), no significant coherences were found in the neural networks of invertebrates, in contrast to the higher coherences between distant structures that were recorded in mammalian and human brains. The highest coherences were found in the subdural structures of the human brain (Bullock 2006). Since coherence is, in essence, a correlation coefficient per frequency band, it is used to describe the coupling or relationship between signals for a certain frequency band. According to Bullock (2003), increased coherence between two structures, namely A and B, can be caused by the following processes: (1) Structures A and B are driven by the same generator; (2) Structures A and B can mutually drive each other; (3) One of the structures, A or B, drives the other.
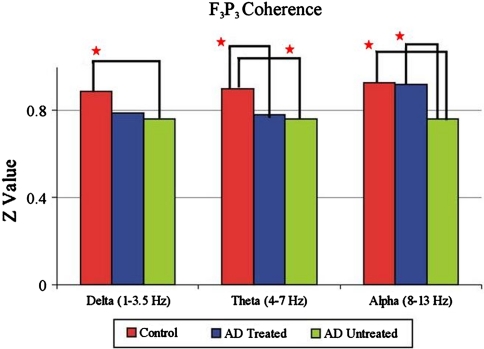
Güntekin et al. (2008) studied the coherence of event related oscillations in patients with Alzheimer type dementia (AD). In responses to a cognitive paradigm delta, theta and alpha coherences were highly reduced in AD patients in comparison to healthy subjects, especially at the left fronto-parietal pair (F3-P3) (Figs. 5 and 6). The highly reduced delta, theta and alpha responses in AD during a cognitive task indicate the disconnection of cortico-cortical pathways, as shown by other methods (Delatour et al. 2004; Leuchter et al. 1992). Abnormal fronto-parietal coupling of brain rhythms in mild AD was also shown by Babiloni et al. (2004). Acetylcholine esterase inhibitors have only a moderate improving effect on coherence, depending on these neural assemblies related to cognitive performance. However, coherence function in lower frequency ranges after a cognitive stimulation seems to be affected more than any other electrophysiological parameter in AD, implying a greater decline in functional connectivity and large-scale networks.
Fig. 5.
Fronto-parietal connectivity (coherence) is decreased in Alzheimer groups relative to control group (n = 19) in delta and theta frequency ranges, whereas the Alzheimer subjects (n = 11) on cholinergic treatment display greater coherence values compared to the untreated Alzheimer group (n = 11). These responses are elicited upon the target stimulation to classical visual oddball paradigm
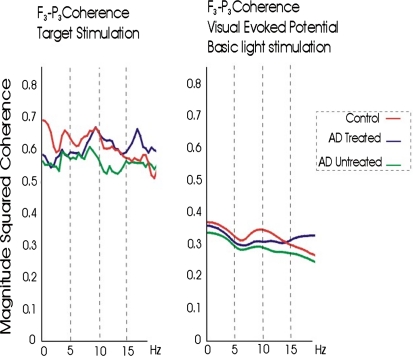
Fig. 6.
Coherences of brain oscillations upon a cognitive task (i.e. target stimulus in classical visual oddball paradigm) reach higher values compared to those elicited upon simple sensory visual stimuli (i.e. basic light stimulation). Coherence reflecting functional connectivity between fronto-parietal regions shows higher values in controls in relation to Alzheimer (AD) subjects. The coherence values in alpha ranges are greater in the cholinergically treated Alzheimer group than those with no treatment, possibly indicating better functional connectivity after cholinergic remedy
Occipital, parietal and prefrontal cortices; visual system; cholinergic mechanisms in Alzheimer’s disease
Understanding how the cholinergic system affects visual sensory or cognitive function is important for Alzheimer’s disease. When two types of tasks, i.e. deep minus shallow visual stimulation, were given to AD patients and controls, fMRI showed that the right parietal (Hao et al. 2005), left prefrontal and superomedial prefrontal cortex were less activated by this task effect in AD patients than in controls (Bentley et al. 2008). The extent of involvement of visual and higher order association cortex increased with greater test complexity in AD. Visual tests activate both primary and secondary visual areas in dorsal stream (Förster et al. 2010). Visual dorsal stream, which involves the parietal lobe, is activated before ventral stream, which includes the temporal lobe. The parietal lobe is activated within 30 ms after occipital activation, occurring at about 56 ms. Visual sensory areas generally continue to be active for 100–400 ms prior to motor output. The feedback processes between sensory, parietal and prefrontal cortices take about 200 ms for an interactive processing. This initial volley of sensory afference through the visual system and involving top-down influences from parietal and frontal regions occur much earlier than the early ERP components (Foxe and Simpson 2002).
Using visuospatial paradigms, these regions are particularly sensitive to cholinergic modulation (Sarter et al. 2001). Acetylcholine seems to have a role in promoting visual-feature detection or signal-to-noise ratios in sensory processing (Hasselmo and Giocomo 2006) and cholinergic medication can improve a normal pattern of task-dependent parietal activation in AD. Working memory tasks (Saykin et al. 2004) or visual search studies (Hao et al. 2005) indicate an enhancement in prefrontal cortex activity after cholinergic medication, similar to the electrophysiological findings of our group (Yener et al. 2007). Recent fMRI studies in mild AD/MCI also showed a similar pattern in left prefrontal regions during attentional demands (Dannhauser et al. 2005). The diffuse innervation of cortical cholinergic neurones (Sarter et al. 2001) can lead to cholinergic modulation in both higher-level (e.g. fronto-parietal) and lower-level (e.g. visual) areas. It is possible that visual event related oscillatory deficits in AD may be related to reduced cholinergic modulation of visual cortex and attention-related frontoparietal cortices (Perry and Hodges 1999).
We believe that task related activity (cognitive versus sensory simple stimuli) in anterior regions have modulating effects over parietal regions and over primary visual regions. Another possible influencing factor may well be the coherences between these regions. Coherences between prefrontal-parietal and prefrontal-occipital regions may have a role in determining the resulting activity in parietal or in occipital regions (Table 1). Our findings are consistent with functional imaging studies in AD, showing a relatively greater attenuation of activations in parieto-occipital (Bentley et al. 2008; Prvulovic et al. 2002; Bradley et al. 2002) than temporo-occipital areas. Cholinesterase inhibitors may improve visual attention dysfunction (Balducci et al. 2003) as also implicated by electrophysiological studies reported by our group (Güntekin et al. 2008; Yener et al. 2007, 2009).
Table 1.
Summary of event related oscillation studies in AD with a comparison to controls
| Cognitive stimuli | Sensory stimuli | ||||
|---|---|---|---|---|---|
| Phase lock | Amplitude | Coherence | Amplitude | Coherence | |
| Yener et al. (2007)* | (−) F3 theta | ||||
| Polikar et al. (2007)* | (−) Pz delta | (−) F3-O1 delta | |||
| Yener et al. (2008)* | (−) C3 delta | (+) F4-T6 beta | |||
| Güntekin et al. (2008)* | (−) F3-P3 alpha | ||||
| (−) F4-P4 alpha | |||||
| (−) F3-O1 alpha | |||||
| (−) F4-O2 delta | |||||
| (−) F3-T5 theta | |||||
| (−) F4-T6 alpha, delta | |||||
| (+) P3, P4, O2 theta | |||||
| Caravaglios et al. (2008)** | (−) all locations delta | ||||
| Yener et al. (2009)* | |||||
Oscillatory responses (* visual, ** auditory)
“(−)” indicates decreased response; “(+)” indicates increased response
Studying medication effects may be achieved by means of electrophysiological methods, especially by oscillatory brain dynamics and coherence. These methods can also provide an opportunity to objectively monitor the medication effects as a frontal enhancement in electrophysiologic responses indicating better cognitive response (Babiloni et al. 2006c) was observed in cholinergically treated AD subjects. However, there is a need for further studies to clarify this matter.
Concluding remarks
The conclusions of the study can be summarized in two different categories.
- Changes of brain dynamics in Alzheimer’s disease.
- Left and mid-central (C3-Cz) areas show decreased delta oscillatory responses in AD subjects. This finding implies poorer cognitive response related to working memory and decision-making in AD.
- Diminished phase locking in theta oscillatory response is observed in the left prefrontal area (F3) of untreated AD group. This indicates a relatively poor cognitive response.
- In all groups, including healthy elderly controls and both AD groups, evoked sensory coherences upon simple light display much lower values in alpha, theta and delta frequency ranges than those in event related responses to a cognitive task, such as an oddball paradigm.
- Upon sensory stimulation, connectivity between left occipital and frontal lobes (F3-O1) are decreased, whereas occipital theta oscillatory responses are increased in AD, contra-intuitively; implying an overattendance in primary visual cortex to simple sensory stimulation. This overattendance may imply a decoupling of frontal and occipital lobes, possibly due to diminished modulation of the occipital lobe by the frontal cortex.
- Upon a cognitive stimulation, the connectivity between left frontal and parietal lobes (F3- P3) is decreased in AD patient compared to healthy controls. Further, as mentioned above, left frontal theta phase locking is decreased in de novo AD group.
- Cholinergic treatment modulates brain oscillatory responses.
- The frontal theta phase locking (i.e. synchronization among single sweeps) is greater in healthy subjects and in the AD subjects treated by cholinergic agents than in the untreated AD group. From the viewpoint of electrophysiology, this finding implies that untreated AD subjects display decreased synchronization over the left frontal area. The treated AD group has a better synchronization and positive response to medication at that specific site (F3).
- The amplitudes of oscillatory responses do not differ between AD subgroups, i.e. those cholinergically treated and de novo groups, implying no effect of cholinergic medication on the amplitudes. Cholinergic agents seem to be more effective on connectivity and synchronization over long distances (coherences) rather than increasing synchronization in local areas (amplitudes) of oscillatory responses in AD.
- In cholinergically treated AD patients, only alpha evoked coherences are increased between left frontal and parietal lobes after cognitive stimulation.
As a consequence of the concluding remarks above, we tentatively assume that the left prefrontal area (F3) has a modulating effect on other parts of the brain, depending on stimulation modality (i.e. sensory or cognitive). However, modulation of the left prefrontal lobe may be different on the projecting areas, depending on the cognitive load of the stimulus. Among the electrophysiological parameters, the event related (or cognitive) coherences comprehend activation of a greater number of neural networks and merit special attention among the assembly of electrophysiological parameters. Therefore, it can be suggested as a candidate electrophysiological biomarker in AD. Other methods, such as phase locking, may also provide insights into cognitive networks and their modulation by neurotransmitter changes.
The pathologically affected regions in AD (i.e. heteromodal association areas) correspond to the default mode network that is active during resting (Raichle and Snyder 2007). Yener et al. (2009) comparing visual sensory and cognitive responses indicate two different networks depending on the type of stimulus. The authors show that sensory evoked oscillations display increased responses, whereas cognitive responses show decreased responses in prefrontal area in AD subjects. Osipova et al. (2006) report similar magnetoencephalography amplitude increase after auditory sensory stimulation over temporal cortex, whereas Caravaglios et al. (2008) indicate decrease in auditory cognitive oscillatory responses over frontal regions in similar group of subjects.
These observations may serve to increase the understanding in physiology of distributed functional networks and, in turn, the possibility of determining biomarkers for either diagnosis or monitoring of medical treatment in AD. It is also important to state that a greater number of subjects are needed to study either the effects of pharmacological applications or its diagnostic role in diseases that alter brain dynamics.
References
- Babiloni C, Binetti G, Cassetta E, et al. Mapping distributed sources of cortical rhythms in mild Alzheimer’s disease. A multicentric EEG study. Neuroimage. 2004;22(1):57–67. doi: 10.1016/j.neuroimage.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Babiloni C, Binetti G, Cassetta E, et al. Sources of cortical rhythms change as a function of cognitive impairment in pathological aging: a multicenter study. Clin Neurophysiol. 2006;117(2):252–268. doi: 10.1016/j.clinph.2005.09.019. [DOI] [PubMed] [Google Scholar]
- Babiloni C, Vecchio F, Bultrini A, et al. Pre- and poststimulus alpha rhythms are related to conscious visual perception a high-resolution EEG study. Cereb Cortex. 2006;16)::1690–1700. doi: 10.1093/cercor/bhj104. [DOI] [PubMed] [Google Scholar]
- Babiloni C, Cassetta E, Dal Forno G, et al. Donepezil effects on sources of cortical rhythms in mild Alzheimer’s disease: responders vs. non-responders. Neuroimage. 2006;31(4):1650–1665. doi: 10.1016/j.neuroimage.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Babiloni C, Cassetta E, Binetti G. Resting EEG sources correlate with attentional span in mild cognitive impairment and Alzheimer’s disease. Eur J Neurosci. 2007;25(12):3742–3757. doi: 10.1111/j.1460-9568.2007.05601.x. [DOI] [PubMed] [Google Scholar]
- Babiloni C, Ferri R, Binetti G. Directionality of EEG synchronization in Alzheimer’s disease subjects. Neurobiol Aging. 2009;30(1):93–102. doi: 10.1016/j.neurobiolaging.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Balducci C, Nurra M, Pietropoli A. Reversal of visual attention dysfunction after AMPA lesions of the nucleus basalis magnocellularis (NBM) by the cholinesterase inhibitor donepezil and by a 5-HT1A receptor antagonist WAY 100635. Psychopharmacology (Berl) 2003;167(1):28–36. doi: 10.1007/s00213-002-1385-7. [DOI] [PubMed] [Google Scholar]
- Başar E. EEG-brain dynamics. Relation between EEG and brain evoked potentials. Amsterdam: Elsevier; 1980. [Google Scholar]
- Başar E. Brain function and oscillations: II. Integrative brain function, neurophysiology and cognitive processes. Berlin: Springer; 1999. [Google Scholar]
- Başar E. Memory and brain dynamics. Oscillations integrating attention, perception, learning and memory. Florida: CRC Press; 2004. [Google Scholar]
- Başar E. The theory of the whole-brain-work. Int J Psychophysiol. 2006;60:133–138. doi: 10.1016/j.ijpsycho.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Başar E, Güntekin B. A review of brain oscillations in cognitive disorders and the role of neurotransmitters. Brain Res. 2008;1235:172–193. doi: 10.1016/j.brainres.2008.06.103. [DOI] [PubMed] [Google Scholar]
- Başar E, Güntekin B, Tülay E, Yener GG (2010) Evoked and event related coherence of alzheimer patients manifest differentiation of sensory-cognitive networks. Brain Res. http://www.dx.doi.org/10.1016/j.brainres.2010.08.054 [DOI] [PubMed]
- Başar-Eroğlu C, Basar E, Demiralp T, Schurmann M. P300-response possible psychophysiological correlates in delta and theta frequency channels A review. Int J Psychophysiol. 1992;13(2):161–179. doi: 10.1016/0167-8760(92)90055-g. [DOI] [PubMed] [Google Scholar]
- Başar-Eroğlu C, Schmiedt-Fehr C, Marbach S, et al. Altered oscillatory alpha and theta networks in schizophrenia. Brain Res. 2008;1235:143–152. doi: 10.1016/j.brainres.2008.06.114. [DOI] [PubMed] [Google Scholar]
- Başar-Eroğlu C, Schmiedt-Fehr C, Mathes B, et al. Are oscillatory brain responses generally reduced in schizophrenia during long sustained attentional processing? Int J Psychophysiol. 2009;71(1):75–83. doi: 10.1016/j.ijpsycho.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Beach TG, McGeer EG. Cholinergic fiber loss occurs in the absence of synaptophysin depletion in Alzheimer’s disease primary visual cortex. Neurosci Lett. 1992;142:253–256. doi: 10.1016/0304-3940(92)90385-k. [DOI] [PubMed] [Google Scholar]
- Beelke M, Sannita WG. Cholinergic function and dysfunction in the visual system. Methods Find Exp Clin Pharmacol. 2002;24:113–117. [PubMed] [Google Scholar]
- Bentley P, Driver J, Dolan RJ. Cholinesterase inhibition modulates visual and attentional brain responses in Alzheimer’s disease and health. Brain. 2008;131(Pt 2):409–424. doi: 10.1093/brain/awm299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E, Bohl J. Staging of Alzheimer-related cortical destruction. Eur Neurol. 1993;33:403–408. doi: 10.1159/000116984. [DOI] [PubMed] [Google Scholar]
- Bradley KM, O’Sullivan VT, Soper ND. Cerebral perfusion SPET correlated with Braak pathological stage in Alzheimer’s disease. Brain. 2002;125(Pt 8):1772–1781. doi: 10.1093/brain/awf185. [DOI] [PubMed] [Google Scholar]
- Bressler SL, Kelso JA. Cortical coordination dynamics and cognition. Trends Cogn Sci. 2001;1:26–36. doi: 10.1016/s1364-6613(00)01564-3. [DOI] [PubMed] [Google Scholar]
- Bruns A. Fourier-, Hilbert- and wavelet-based signal analysis: are they really different approaches? J Neurosci Methods. 2004;137(2):321–332. doi: 10.1016/j.jneumeth.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Snyder AZ, Shannon BJ, et al. Molecular, structural and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25:7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock TH. Have brain dynamics evolved? Should we look for unique dynamics in the sapient species? Neural Comput. 2003;9:2013–2027. doi: 10.1162/089976603322297269. [DOI] [PubMed] [Google Scholar]
- Bullock TH. How do brains evolve complexity? An essay. Int J Psychophysiol. 2006;60:106–109. doi: 10.1016/j.ijpsycho.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Bullock TH, Basar E. Comparison of ongoing compound field potentials in the brain of inver tebrates and vertebrates. Brain Res Rev. 1988;13:57–75. doi: 10.1016/0165-0173(88)90005-7. [DOI] [PubMed] [Google Scholar]
- Bullock TH, McClune MC, Achimowicz JZ, et al. EEG coherence has structure in the millimeter domain: subdural and hippocampal recordings from epileptic patients. Electroencephalogr Clin Neurophysiol. 1995;95:161–177. doi: 10.1016/0013-4694(95)93347-a. [DOI] [PubMed] [Google Scholar]
- Buscema M, Grossi E, Capriotti M, et al. The I.F.A.S.T. model allows the prediction of conversion to Alzheimer disease in patients with mild cognitive impairment with high degree of accuracy. Curr Alzheimer Res. 2010;2:173–187. doi: 10.2174/156720510790691137. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Theta oscillations in the hippocampus. Neuron. 2002;33:325–340. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- Buzsaki G (2006) Rhythms of the brain. Oxford University Press
- Caravaglios G, Costanzo E, Palermo F, Muscoso EG. Decreased amplitude of auditory event-related delta responses in Alzheimer’s disease. Int J Psychophysiol. 2008;70(1):23–32. doi: 10.1016/j.ijpsycho.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Cichocki A, Shishkin SL, Musha T. EEG filtering based on blind source separation (BSS) for early detection of Alzheimer’s disease. Clin Neurophysiol. 2005;116:729–737. doi: 10.1016/j.clinph.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Dannhauser TM, Walker Z, Stevens T. The functional anatomy of divided attention in amnestic mild cognitive impairment. Brain. 2005;128(Pt 6):1418–1427. doi: 10.1093/brain/awh413. [DOI] [PubMed] [Google Scholar]
- Dauwels J, Vialatte F, Latchoumane C et al (2009) EEG synchrony analysis for early diagnosis of Alzheimer’s disease: a study with several synchrony measures and EEG data sets. Conf Proc IEEE Eng Med Biol Soc :2224–2227 [DOI] [PubMed]
- Dauwels J, Vialatte F, Musha T, Cichocki A. A comparative study of synchrony measures for the early diagnosis of Alzheimer’s disease based on EEG. Neuroimage. 2010;49(1):668–693. doi: 10.1016/j.neuroimage.2009.06.056. [DOI] [PubMed] [Google Scholar]
- Davis KL, Mohs RC, Marin D, et al. Cholinergic markers in elderly patients with early signs of Alzheimer’s disease. JAMA. 1999;281:1401–1406. doi: 10.1001/jama.281.15.1401. [DOI] [PubMed] [Google Scholar]
- Haan W, Stam CJ, Jones BF, et al. Resting-state oscillatory brain dynamics in Alzheimer disease. J Clin Neurophysiol. 2008;25(4):187–193. doi: 10.1097/WNP.0b013e31817da184. [DOI] [PubMed] [Google Scholar]
- Delatour B, Blanchard V, Pradier L, et al. Alzheimer pathology disorganizes cortico–cortical circuitry: direct evidence from a transgenic animal model. Neurobiol Dis. 2004;16(1):41–47. doi: 10.1016/j.nbd.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Demiralp T, Başar-Eroglu C, Rahn E, et al. Event-related theta rhythms in cat hippocampus and prefrontal cortex during an omitted stimulus paradigm. Int J Psychophysiol. 1994;18(1):35–48. doi: 10.1016/0167-8760(84)90013-8. [DOI] [PubMed] [Google Scholar]
- Drzezga A, Lautenschlager N, Siebner H, et al. Cerebral metabolic changes accompanying conversion of mild cognitive impairment into Alzheimer’s disease: a PET follow-up study. Eur J Nucl Med Mol Imag. 2003;30:1104–1113. doi: 10.1007/s00259-003-1194-1. [DOI] [PubMed] [Google Scholar]
- Espeseth T, Endestad T, Rootwelt H, Reinvang I. Nicotine receptor gene CHRNA4 modulates early event-related potentials in auditory and visual oddball target detection tasks. Neuroscience. 2007;147(4):974–985. doi: 10.1016/j.neuroscience.2007.04.027. [DOI] [PubMed] [Google Scholar]
- Ferri CP, Prince M, Brayne C, et al. Alzheimer’s disease international. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366(9503):2112–2117. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Förster S, Teipel S, Zach C, et al. FDG-PET mapping the brain substrates of visuo-constructive processing in Alzheimer s disease. J Psychiatr Res. 2010;44(7):462–469. doi: 10.1016/j.jpsychires.2009.09.012. [DOI] [PubMed] [Google Scholar]
- Foxe JJ, Simpson GV. Flow of activation from V1 to frontal cortex in humans. A framework for defining “early” visual processing. Exp Brain Res. 2002;142(1):139–150. doi: 10.1007/s00221-001-0906-7. [DOI] [PubMed] [Google Scholar]
- Gardner W. A unifying view of coherence in signal processing. Signal Process. 1992;29(2):113–140. [Google Scholar]
- Güntekin B, Başar E. A new interpretation of P300 responses upon analysis of coherences. Cogn Neurodyn. 2010;4:107–118. doi: 10.1007/s11571-010-9106-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güntekin B, Saatçi E, Yener G. Decrease of evoked delta, theta and alpha coherence in Alzheimer patients during a visual oddball paradigm. Brain Res. 2008;1235:109–116. doi: 10.1016/j.brainres.2008.06.028. [DOI] [PubMed] [Google Scholar]
- Hao J, Li K, Li K, et al. Visual attention deficits in Alzheimer’s disease: an fMRI study. Neurosci Lett. 2005;385(1):18–23. doi: 10.1016/j.neulet.2005.05.028. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Giocomo LM. Cholinergic modulation of cortical function. J Mol Neurosci. 2006;30(12):133–136. doi: 10.1385/JMN:30:1:133. [DOI] [PubMed] [Google Scholar]
- Haupt M, González-Hernández JA, Scherbaum WA. Regions with different evoked frequency band responses during early-stage visual processing distinguish mild Alzheimer dementia from mild cognitive impairment and normal aging. Neurosci Lett. 2008;442(3):273–278. doi: 10.1016/j.neulet.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Herrmann CS, Demiralp T. Human EEG gamma oscillations in neuropsychiatric disorders. Clin Neuropsysiol. 2005;116(12):2719–2733. doi: 10.1016/j.clinph.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Hof PR, Morrison JH. Quantitative analysis of a vulnerable subset of pyramidal neurons in Alzheimer’s disease: II. Primary and secondary visual cortex. J Comp Neurol. 1990;301:55–64. doi: 10.1002/cne.903010106. [DOI] [PubMed] [Google Scholar]
- Hogan MJ, Swanwick GR, Kaiser J, et al. Memory-related EEG power and coherence reductions in mild Alzheimer’s disease. Int J Psychophysiol. 2003;49:147–163. doi: 10.1016/s0167-8760(03)00118-1. [DOI] [PubMed] [Google Scholar]
- Jackson CE, Snyder PJ. EEG and ERP as biomarkers of mild cognitive impairment and mild Alzheimer’s disease. Alzheimer’s Dementia. 2008;4:S137–S143. doi: 10.1016/j.jalz.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Jelles B, Birgelen JH, Slaets JP, et al. Decrease of non-linear structure in the EEG of Alzheimer patients compared to healthy controls. Clin Neurophysiol. 1999;110(7):1159–1167. doi: 10.1016/s1388-2457(99)00013-9. [DOI] [PubMed] [Google Scholar]
- Jeong J. EEG dynamics in patients with Alzheimer’s disease. Clin Neurophysiol. 2004;115(7):1490–1505. doi: 10.1016/j.clinph.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Jones KA, Porjesz B, Almasy L, et al. A cholinergic receptor gene (CHRM2) affects event-related oscillations. Behav Genet. 2006;36(5):627–639. doi: 10.1007/s10519-006-9075-6. [DOI] [PubMed] [Google Scholar]
- Kahana MJ, Sekuler R, Caplan JB, et al. Human theta oscillations exhibit task dependence during virtual maze navigation. Nature. 1999;399:781–784. doi: 10.1038/21645. [DOI] [PubMed] [Google Scholar]
- Karakaş S, Erzengin OU, Başar E. A new strategy involving multiple cognitive paradigms demonstrates that ERP components are determined by the superposition of oscillatory responses. Clin Neurophysiol. 2000;111:1719–1732. doi: 10.1016/s1388-2457(00)00418-1. [DOI] [PubMed] [Google Scholar]
- Karrasch M, Laine M, Rinne JO, et al. Brain oscillatory responses to an auditory-verbal working memory task in mild cognitive impairment and Alzheimer’s disease. Int J Psychophysiol. 2006;59(2):168–178. doi: 10.1016/j.ijpsycho.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Kawas CH, Corrada MM, Brookmeyer R, et al. Visual memory predicts Alzheimer’s disease more than a decade before diagnosis. Neurology. 2003;60:1089–1093. doi: 10.1212/01.wnl.0000055813.36504.bf. [DOI] [PubMed] [Google Scholar]
- Keskinoğlu P, Giray H, Picakciefe M, et al. The prevalence and risk factors of dementia in the elderly population in a low socio-economic region of Izmir, Turkey. Arch Gerontol Geriatr. 2006;43(1):93–100. doi: 10.1016/j.archger.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Schack B, Schabus M, et al. Phase-locked alpha and theta oscillations generate the P1–N1 complex and are related to memory performance. Cogn Brain Res. 2004;19:302–316. doi: 10.1016/j.cogbrainres.2003.11.016. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Freunberger R, Sauseng P. A short review of slow phase synchronisation and memory: evidence for control processes in different memory systems? Brain Res. 2008;1235:31–44. doi: 10.1016/j.brainres.2008.06.049. [DOI] [PubMed] [Google Scholar]
- Kukleta M, Bob P, Brázdil M, et al. Beta 2-band synchronization during a visual oddball task. Physiol Res. 2009;58(5):725–732. doi: 10.33549/physiolres.931629. [DOI] [PubMed] [Google Scholar]
- Lachaux JP, Lutz A, Rudrauf D, et al. Estimating the time-course of coherence between single-trial brain signals: an introduction to wavelet coherence. Neurophysiol Clin. 2002;32(3):157–174. doi: 10.1016/s0987-7053(02)00301-5. [DOI] [PubMed] [Google Scholar]
- Leuchter AF, Newton TF, Cook IA, et al. Changes in brain functional connectivity in Alzheimer-type and multi-infarct dementia. Brain. 1992;115:1543–1561. doi: 10.1093/brain/115.5.1543. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Campbell MJ, Terry RD, et al. Laminar and regional distributions of neurofibrillary tangles and neuritic plaques in Alzheimer’s disease: a quantitative study of visual and auditory cortices. J Neurosci. 1987;7:1799–1808. doi: 10.1523/JNEUROSCI.07-06-01799.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes da Silva FH, Vos JE, Mooibroek J, et al. Relative contributions of intracortical and thalamo-cortical processes in the generation of alpha rhythms, revealed by partial coherence analysis. Electroencephalogr Clin Neurophysiol. 1980;50(5–6):449–450. doi: 10.1016/0013-4694(80)90011-5. [DOI] [PubMed] [Google Scholar]
- Maltseva I, Geissler HG, Başar E. Alpha oscillations as an indicator dynamic memory operations: anticipation of omitted stimuli. Int J Psychophysiol. 2000;36:185–197. doi: 10.1016/s0167-8760(99)00093-8. [DOI] [PubMed] [Google Scholar]
- McKee AC, Au R, Cabral HJ, et al. Visual association pathology in preclinical Alzheimer disease. J Neuropathol Exp Neurol. 2006;65(6):621–630. doi: 10.1097/00005072-200606000-00010. [DOI] [PubMed] [Google Scholar]
- Missonnier P, Gold G, Herrmann FR, et al. Decreased theta event-related synchronization during working memory activation is associated with progressive mild cognitive impairment. Dement Geriatr Cogn Disord. 2006;22(3):250–259. doi: 10.1159/000094974. [DOI] [PubMed] [Google Scholar]
- Monacelli AM, Cushman LA, Kavcic V, et al. Spatial disorientation in Alzheimer’s disease: the remembrance of things passed. Neurology. 2003;61:1491–1497. doi: 10.1212/wnl.61.11.1491. [DOI] [PubMed] [Google Scholar]
- Morrison JH, Lewis DA, Campbell MJ, et al. A monoclonal antibody to non-phosphorylated neurofilament protein marks the vulnerable cortical neurons in Alzheimer’s disease. Brain Res. 1987;416:331–336. doi: 10.1016/0006-8993(87)90914-0. [DOI] [PubMed] [Google Scholar]
- Morrison JH, Hof PR, Bouras C. An anatomic substrate for visual disconnection in Alzheimer’s disease. Ann NY Acad Sci. 1991;640:36–43. doi: 10.1111/j.1749-6632.1991.tb00187.x. [DOI] [PubMed] [Google Scholar]
- Nunez PL. EEG coherence measures in medical and cognitive science: a general overview of experimental methods, computer algorithms, and accuracy. In: Eselt M, Zwiener U, Witte H, editors. Quantative and topological EEG and MEG analysis. Jena: Universitätsverlag Druckhaus Mayer; 1997. [Google Scholar]
- Osipova D, Pekkonen E, Ahveninen J. Enhanced magnetic auditory steady-state response in early Alzheimer’s disease. Clin Neurophysiol. 2006;117(9):1990–1995. doi: 10.1016/j.clinph.2006.05.034. [DOI] [PubMed] [Google Scholar]
- Özerdem A, Güntekin B, Tunca Z, et al. Brain oscillatory responses in patients with bipolar disorder manic episode before and after valproate treatment. Brain Res. 2008;1235:98–108. doi: 10.1016/j.brainres.2008.06.101. [DOI] [PubMed] [Google Scholar]
- Özerdem A, Kocaaslan S, Tunca Z, et al. Event related oscillations in euthymic patients with bipolar disorder. Neurosci Lett. 2008;444(1):5–10. doi: 10.1016/j.neulet.2008.07.081. [DOI] [PubMed] [Google Scholar]
- Özerdem A, Güntekin B, Saatçi E et al (2010) Disturbance in long distance gamma coherence in bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry 34(6):861–865 [DOI] [PubMed]
- Pariente J, Cole S, Henson R, et al. Alzheimer’s patients engage an alternative network during a memory task. Ann Neurol. 2005;58(6):870–879. doi: 10.1002/ana.20653. [DOI] [PubMed] [Google Scholar]
- Perry RJ, Hodges JR. Attention and executive deficits in Alzheimer’s disease A critical review. Brain. 1999;122(Pt 3):383–404. doi: 10.1093/brain/122.3.383. [DOI] [PubMed] [Google Scholar]
- Petsche H, Etlinger SC. EEG and thinking: power and coherence analysis of cognitive processes. Wien: Verlag Der Österreichischen Akademie Der Wissenscaften; 1998. [Google Scholar]
- Polich J, Herbst KL. P300 as a clinical assay: rationale, evaluation, and findings. Int J Psychophysiol. 2000;38:3–19. doi: 10.1016/s0167-8760(00)00127-6. [DOI] [PubMed] [Google Scholar]
- Polikar R, Topalis A, Green D, et al. Comparative multiresolution wavelet analysis of ERP spectral bands using an ensemble of classifiers approach for early diagnosis of Alzheimer’s disease. Comput Biol Med. 2007;37(4):542–558. doi: 10.1016/j.compbiomed.2006.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prvulovic D, Hubl D, Sack AT, et al. Functional imaging of visuospatial processing in Alzheimer’s disease. Neuroimage. 2002;17(3):1403–1414. doi: 10.1006/nimg.2002.1271. [DOI] [PubMed] [Google Scholar]
- Raichle ME, Snyder AZ. A default mode of brain function: a brief history of an evolving idea. Neuroimage. 2007;37(4):1083–1090. doi: 10.1016/j.neuroimage.2007.02.041. [DOI] [PubMed] [Google Scholar]
- Rappelsberger P, Pockberger H. EEG-Veranderungen beim Lesen. In: Weinmann HM, editor. Zugang zum Verstandnis hoherer Hirnfunktionen durch das EEG. Munchen: Zuckschwerdt; 1987. pp. 59–74. [Google Scholar]
- Rossini PM, Del Percio C, Pasqualetti P, et al. Conversion from mild cognitive impairment to Alzheimer’s disease is predicted by sources and coherence of brain electroencephalography rhythms. Neuroscience. 2006;143(3):793–803. doi: 10.1016/j.neuroscience.2006.08.049. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Buscema M, Capriotti M, et al. Is it possible to automatically distinguish resting EEG data of normal elderly vs. mild cognitive impairment subjects with high degree of accuracy? Clin Neurophysiol. π;119(7):1534–1545. doi: 10.1016/j.clinph.2008.03.026. [DOI] [PubMed] [Google Scholar]
- Sarter M, Givens B, Bruno JP. The cognitive neuroscience of sustained attention: where top-down meets bottom-up. Brain Res Brain Res Rev. 2001;35(2):146–160. doi: 10.1016/s0165-0173(01)00044-3. [DOI] [PubMed] [Google Scholar]
- Saykin AJ, Wishart HA, Rabin LA. Cholinergic enhancement of frontal lobe activity in mild cognitive impairment. Brain. 2004;127:1574–1583. doi: 10.1093/brain/awh177. [DOI] [PubMed] [Google Scholar]
- Stepanyants A, Hof PR, Chklovskii DB. Geometry and structural plasticity of synaptic connectivity. Neuron. 2002;34:275–288. doi: 10.1016/s0896-6273(02)00652-9. [DOI] [PubMed] [Google Scholar]
- Stern Y, Moeller JR, Anderson KE, et al. Different brain networks mediate task performance in normal aging and AD: defining compensation. Neurology. 2000;55(9):1291–1297. doi: 10.1212/wnl.55.9.1291. [DOI] [PubMed] [Google Scholar]
- Tsao DY, Freiwald WA, Knutsen TA, et al. Faces and objects in macaque cerebral cortex. Nature Neurosci. 2003;6:989–995. doi: 10.1038/nn1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela FJ. Guiding the study of brain dynamics by using first-person data: synchrony patterns correlate with ongoing conscious states during a simple visual task. Proc Natl Acad Sci USA. 2002;99(3):1586–1591. doi: 10.1073/pnas.032658199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela F, Lachaux JP, Rodriguez E, et al. The brainweb: phase syncronization and large-scale integration. Nat Rev Neurosci. 2001;2:229–232. doi: 10.1038/35067550. [DOI] [PubMed] [Google Scholar]
- Vialatte FB, Dauwels J, Maurice M, et al. On the synchrony of steady state visual evoked potentials and oscillatory burst events. Cogn Neurodyn. 2009;3(3):251–261. doi: 10.1007/s11571-009-9082-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein A, Sarnthein J. Different frequencies for different scales of cortical integration: from local gamma to long range alpha/theta synchronization. Int J Psychophysiol. 2000;38(3):301–313. doi: 10.1016/s0167-8760(00)00172-0. [DOI] [PubMed] [Google Scholar]
- Yener GG, Leuchter AF, Jenden D, et al. Quantitative EEG in frontotemporal dementia. Clin Electroencephal. 1996;27(2):61–68. doi: 10.1177/155005949602700204. [DOI] [PubMed] [Google Scholar]
- Yener GG, Güntekin B, Öniz A, et al. Increased frontal phase-locking of event-related theta oscillations in Alzheimer patients treated with cholinesterase inhibitors. Int J Psychophysiol. 2007;64(1):46–52. doi: 10.1016/j.ijpsycho.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Yener G, Güntekin B, Başar E. Event related delta oscillatory responses of Alzheimer patients. Eur J Neurology. 2008;15(6):540–547. doi: 10.1111/j.1468-1331.2008.02100.x. [DOI] [PubMed] [Google Scholar]
- Yener G, Güntekin B, Tülay E, et al. A comparative analysis of sensory visual evoked oscillations with visual cognitive event related oscillations in Alzheimer’s disease. Neurosci Lett. 2009;462:193–197. doi: 10.1016/j.neulet.2009.07.036. [DOI] [PubMed] [Google Scholar]
- Zeki S, Watson JD, Lueck CJ, et al. A direct demonstration of functional specialization in human visual cortex. J Neurosci. 1991;11(3):641–649. doi: 10.1523/JNEUROSCI.11-03-00641.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng-yan J. Abnormal cortical functional connections in Alzheimer’s disease: analysis of inter-and intra-hemispheric EEG coherence. J Zhejiang Univ SCI. 2005;6B(4):259–264. doi: 10.1631/jzus.2005.B0259. [DOI] [PMC free article] [PubMed] [Google Scholar]