Table II.
Molar Heat Capacities of the Solvate Formation 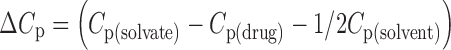
| Heat capacity at 25°C (J g−1 K−1) | Molar heat capacity at 25°C (J/(mol K) | Molar heat capacity of solvent (J/(mol K) (24) | ΔC p at 25°C (J/(mol K) | |
|---|---|---|---|---|
| Anhydrous nevirapine sample (NEV) | 1.55 ± 0.014 | 412.76 ± 0.11 | – | – |
| Ethanolate (Form I) NEV+ (0.5) C2H5OH | 1.66 ± 0.024 | 480.29 ± 0.05 | 112.24 | 11.41 |
| Acetonitrilate (Form II)NEV + (0.5) CH3CN | 1.74 ± 0.003 | 499.07 ± 0.04 | 91.70 | 40.46 |
| Chloroformate (Form III) NEV+ (0.5)CHCl3 | 1.60 ± 0.027 | 520.61 ± 0.02 | 114.25 | 50.73 |
| THF solvate (Form IV)NEV+ (0.5) THF | 2.04 ± 0.004 | 616.79 ± 0.12 | 123.00 | 142.53 |
| Mixed solvate of ethanol and water (Form V) NEV+ C2H5OH(0.5)+ H2O(0.5) | 2.05 ± 0.019 | 611.59 ± 0.12 | 187.48 | 105.09 |
| Toluene solvate (Form VI) NEV+ (0.5)Toluene | 1.63 ± 0.007 | 509.16 ± 0.05 | 155.96 | 18.42 |
