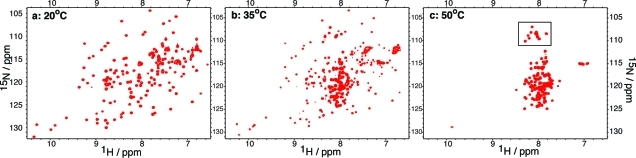
Figure 2.
1H−15N HSQC spectra of I59T at pH 1.2 recorded at different temperatures. At 20 °C, the protein is fully folded (a), whereas at 50 °C, the protein is fully unfolded (c). Near the midpoint of the unfolding transition, at 35 °C, the protein shows resonances typical of both folded and unfolded states (b). The region boxed in panel (c) indicates the peaks corresponding to glycine residues in the unfolded state.